
A Publication Organic of Reliable Methods for the Preparation yntheses of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry.All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as dent Practices n the Laboratory"(The National Academies Press,Washington,D.C. 2011;the full text can be accessed free of charge http://www.nap.edu/catalog.php?record_id=12654).All chemical waste should be disposed of in accordance with local regulations.For general guidelines for the management of chemical waste,see Chapter 8 of Prudent Practices. chemical-pecific hazards are highighted in re It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure.Prior to performing a reaction,a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for out asses and for analy vzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk.Organic Syntheses,Inc.,its Editors,and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have sulted from or related in any way to the procedures herein These paragraphs were added in September 2014 The statements above do not supersede any specific nazard caution notes and satety instructions included in the procedure
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. These paragraphs were added in September 2014. The statements above do not supersede any specific hazard caution notes and safety instructions included in the procedure
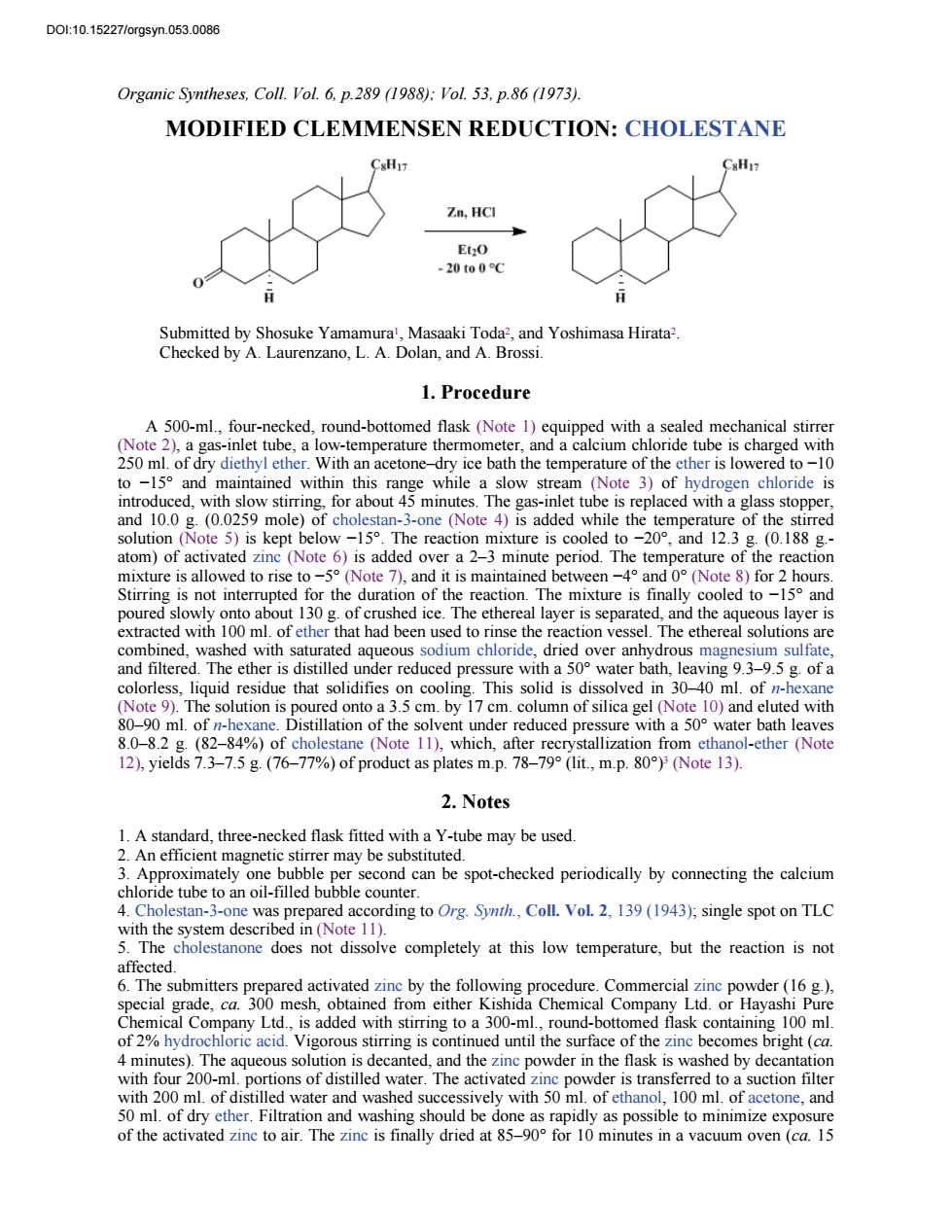
D0t10.152271 orgsyn.053.0086 Organic Syntheses.Coll.Vol.6.p.289(1988):Vol.53.p.86(1973). MODIFIED CLEMMENSEN REDUCTION:CHOLESTANE &HI Zn.HCl 20t00 Submitted by Shosuke Yamamura,Masaaki Toda,and Yoshimasa Hirata2. Checked by A.Laurenzano.L.A.Dolan.and A.Brossi. 1.Procedure A 500-ml.four-necked,round-bottomed flask (Note 1)equipped with a sealed mechanical stirre tub to -159 and maintained within this range while a slowst Note3 of hydrogen chloride is introduced,with slow stirring,for about 45 minutes.The gas-inlet tube is replaced with a glass stopper and mole)ochosa-one ( (Note 6)is added o ter mixture is allowed to rise to-(Note 7).and it is maintained between4 and (Note 8)for 2 hours Stirring is not interrupted for the duration of the reaction.The mixture is finally cooled to-15 and poured slowly ont bout of crus ed ice.T e ethereal layer is separate and he aqueous s layer is omined washed with satura queous sodium chloride and filtered.The ether is distilled under reduced pressure with a5 water bath.leaving939.g of a on solid is dis oo ml.of onto a 8082 g.(82-84%)of cholestane (Note which.after from thanol-ther Note 12).yields 7.3-7.5g.(76-77%)of product as plates m.p.78-79(lit.,m.p.80)(Note 13). 2.Notes 1.A standard,three-necked flask fitted with a Y-tube may be used An efficient magnetic stirrer may be substituted. imately one can be spot-checked periodically by connecting the calcium oe tCal Vat 21 with the system described in(Note 11). The cholestanone does not dissolve completely at this low temperature,but the reaction is not 宽点品品 ydro igorous stirring is e on the zinc be with four 200 ons of distilled water The activated zin wder is transfer red to a suction filte with 200 ml.of distilled water and washed successively with 50ml.of ethanol,100 ml.of acetone,and
Organic Syntheses, Coll. Vol. 6, p.289 (1988); Vol. 53, p.86 (1973). MODIFIED CLEMMENSEN REDUCTION: CHOLESTANE Submitted by Shosuke Yamamura1, Masaaki Toda2, and Yoshimasa Hirata2. Checked by A. Laurenzano, L. A. Dolan, and A. Brossi. 1. Procedure A 500-ml., four-necked, round-bottomed flask (Note 1) equipped with a sealed mechanical stirrer (Note 2), a gas-inlet tube, a low-temperature thermometer, and a calcium chloride tube is charged with 250 ml. of dry diethyl ether. With an acetone–dry ice bath the temperature of the ether is lowered to −10 to −15° and maintained within this range while a slow stream (Note 3) of hydrogen chloride is introduced, with slow stirring, for about 45 minutes. The gas-inlet tube is replaced with a glass stopper, and 10.0 g. (0.0259 mole) of cholestan-3-one (Note 4) is added while the temperature of the stirred solution (Note 5) is kept below −15°. The reaction mixture is cooled to −20°, and 12.3 g. (0.188 g.- atom) of activated zinc (Note 6) is added over a 2–3 minute period. The temperature of the reaction mixture is allowed to rise to −5° (Note 7), and it is maintained between −4° and 0° (Note 8) for 2 hours. Stirring is not interrupted for the duration of the reaction. The mixture is finally cooled to −15° and poured slowly onto about 130 g. of crushed ice. The ethereal layer is separated, and the aqueous layer is extracted with 100 ml. of ether that had been used to rinse the reaction vessel. The ethereal solutions are combined, washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and filtered. The ether is distilled under reduced pressure with a 50° water bath, leaving 9.3–9.5 g. of a colorless, liquid residue that solidifies on cooling. This solid is dissolved in 30–40 ml. of n-hexane (Note 9). The solution is poured onto a 3.5 cm. by 17 cm. column of silica gel (Note 10) and eluted with 80–90 ml. of n-hexane. Distillation of the solvent under reduced pressure with a 50° water bath leaves 8.0–8.2 g. (82–84%) of cholestane (Note 11), which, after recrystallization from ethanol-ether (Note 12), yields 7.3–7.5 g. (76–77%) of product as plates m.p. 78–79° (lit., m.p. 80°)3 (Note 13). 2. Notes 1. A standard, three-necked flask fitted with a Y-tube may be used. 2. An efficient magnetic stirrer may be substituted. 3. Approximately one bubble per second can be spot-checked periodically by connecting the calcium chloride tube to an oil-filled bubble counter. 4. Cholestan-3-one was prepared according to Org. Synth., Coll. Vol. 2, 139 (1943); single spot on TLC with the system described in (Note 11). 5. The cholestanone does not dissolve completely at this low temperature, but the reaction is not affected. 6. The submitters prepared activated zinc by the following procedure. Commercial zinc powder (16 g.), special grade, ca. 300 mesh, obtained from either Kishida Chemical Company Ltd. or Hayashi Pure Chemical Company Ltd., is added with stirring to a 300-ml., round-bottomed flask containing 100 ml. of 2% hydrochloric acid. Vigorous stirring is continued until the surface of the zinc becomes bright (ca. 4 minutes). The aqueous solution is decanted, and the zinc powder in the flask is washed by decantation with four 200-ml. portions of distilled water. The activated zinc powder is transferred to a suction filter with 200 ml. of distilled water and washed successively with 50 ml. of ethanol, 100 ml. of acetone, and 50 ml. of dry ether. Filtration and washing should be done as rapidly as possible to minimize exposure of the activated zinc to air. The zinc is finally dried at 85–90° for 10 minutes in a vacuum oven (ca. 15 DOI:10.15227/orgsyn.053.0086
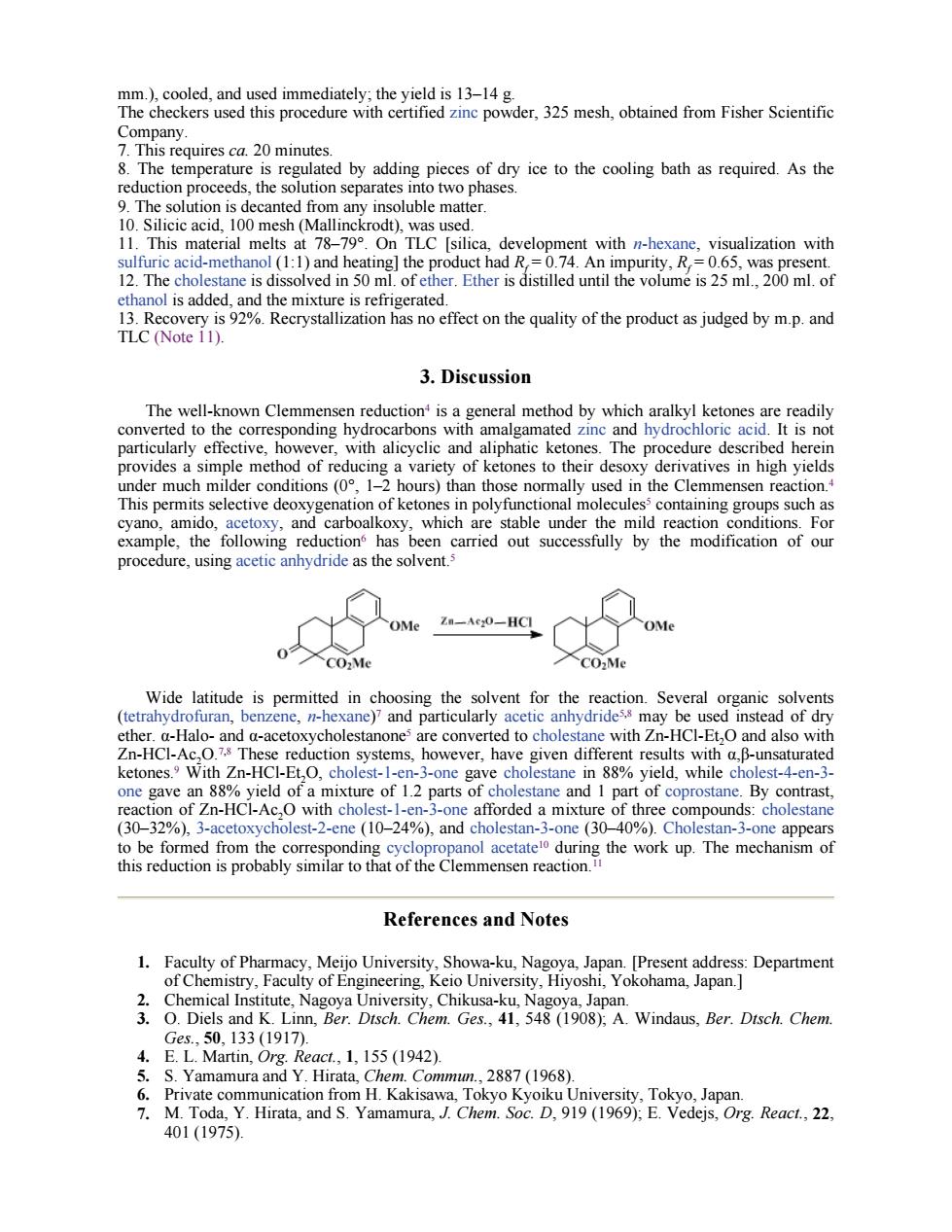
Te th品。 der,325 mesh,obtained from Fisher Scientific rquires c 20 minutes 8.The temperature is regulated by adding pieces of dry ice to the cooling bath as required.As the reduction proceeds,the solution separates in to two phases. e matter 1.This material melts at 7 OnTLC silica.development with nhexane,visualization with The cho dissolved in 50 ml.of is adde effect on the quality of the product as judged by m.p.and TLC (Note i1). 3.Discussion The well-known Clemmensen reduction is a general method by which aralkyl ketones are readily converted to the corresponding hydrocarbons with amalgamated zinc and hydro chloric acid.It is not ularly effe wev e Ine procedure des d her in the ve ditions (08 e This permits selective deoxygenation of ketones in polyfunctional molecules containing grou s such as carboalkoxy,which stable under the mild reactior onditions.For reductio proce e,using acetic anhydride as the solver OMe Zn-Ac:0-HC1 人OM 0 enahydoatmuee Wide l is permitted in anding the ether.a-Halo-and a-acetoxycholestanone are converted to cholestane with Zn-HCI-Et,O and also with Zn-HCl-Ac,O.7 These reduction systems,however,have given different results with a,B-unsaturated Ketones.W Zn-HCl-Et,O,cholest- 3-one stane in 88%yield,while cho st-4-en- B f thre nds- (30-32%).3-acetoxycholest-2-ene(10-24%),and cholestan-3-one(3040%).Cholestan-3-one appears References and Notes 3 Ges.,50,133(1917). 4.E.L.Martin,Org.React.,1,155 (1942). Yamamura. 1.Commun. 2887(1968) M.Toda Y.Hrata. and S.Ya ra.Ch D,919969 401(1975)
mm.), cooled, and used immediately; the yield is 13–14 g. The checkers used this procedure with certified zinc powder, 325 mesh, obtained from Fisher Scientific Company. 7. This requires ca. 20 minutes. 8. The temperature is regulated by adding pieces of dry ice to the cooling bath as required. As the reduction proceeds, the solution separates into two phases. 9. The solution is decanted from any insoluble matter. 10. Silicic acid, 100 mesh (Mallinckrodt), was used. 11. This material melts at 78–79°. On TLC [silica, development with n-hexane, visualization with sulfuric acid-methanol (1:1) and heating] the product had Rf = 0.74. An impurity, Rf = 0.65, was present. 12. The cholestane is dissolved in 50 ml. of ether. Ether is distilled until the volume is 25 ml., 200 ml. of ethanol is added, and the mixture is refrigerated. 13. Recovery is 92%. Recrystallization has no effect on the quality of the product as judged by m.p. and TLC (Note 11). 3. Discussion The well-known Clemmensen reduction4 is a general method by which aralkyl ketones are readily converted to the corresponding hydrocarbons with amalgamated zinc and hydrochloric acid. It is not particularly effective, however, with alicyclic and aliphatic ketones. The procedure described herein provides a simple method of reducing a variety of ketones to their desoxy derivatives in high yields under much milder conditions (0°, 1–2 hours) than those normally used in the Clemmensen reaction.4 This permits selective deoxygenation of ketones in polyfunctional molecules5 containing groups such as cyano, amido, acetoxy, and carboalkoxy, which are stable under the mild reaction conditions. For example, the following reduction6 has been carried out successfully by the modification of our procedure, using acetic anhydride as the solvent.5 Wide latitude is permitted in choosing the solvent for the reaction. Several organic solvents (tetrahydrofuran, benzene, n-hexane)7 and particularly acetic anhydride5,8 may be used instead of dry ether. α-Halo- and α-acetoxycholestanone5 are converted to cholestane with Zn-HCl-Et2O and also with Zn-HCl-Ac2O.7,8 These reduction systems, however, have given different results with α,β-unsaturated ketones.9 With Zn-HCl-Et2O, cholest-1-en-3-one gave cholestane in 88% yield, while cholest-4-en-3- one gave an 88% yield of a mixture of 1.2 parts of cholestane and 1 part of coprostane. By contrast, reaction of Zn-HCl-Ac2O with cholest-1-en-3-one afforded a mixture of three compounds: cholestane (30–32%), 3-acetoxycholest-2-ene (10–24%), and cholestan-3-one (30–40%). Cholestan-3-one appears to be formed from the corresponding cyclopropanol acetate10 during the work up. The mechanism of this reduction is probably similar to that of the Clemmensen reaction.11 References and Notes 1. Faculty of Pharmacy, Meijo University, Showa-ku, Nagoya, Japan. [Present address: Department of Chemistry, Faculty of Engineering, Keio University, Hiyoshi, Yokohama, Japan.] 2. Chemical Institute, Nagoya University, Chikusa-ku, Nagoya, Japan. 3. O. Diels and K. Linn, Ber. Dtsch. Chem. Ges., 41, 548 (1908); A. Windaus, Ber. Dtsch. Chem. Ges., 50, 133 (1917). 4. E. L. Martin, Org. React., 1, 155 (1942). 5. S. Yamamura and Y. Hirata, Chem. Commun., 2887 (1968). 6. Private communication from H. Kakisawa, Tokyo Kyoiku University, Tokyo, Japan. 7. M. Toda, Y. Hirata, and S. Yamamura, J. Chem. Soc. D, 919 (1969); E. Vedejs, Org. React., 22, 401 (1975)

8.S.Yamamura,Chem.Commun.,1494(1968). M.1 Eaand amamura.Bul Chem.Soc.Jpn.45.264( elkin and P O.Rev.CLondon)23 522 (1969)and refa Appendix Chemical Abstracts Nomenclature(Collective Index Number); (Kegistry Number) ethanol(64-17-5) Benzene (71-43-2) the diethyl ether(60-29-7) acetic anhydride(108-24-7) sodium chloride(7647-14-5) acetone(67-64-1) z1nc(7440-66-6) magnesium sulfate(7487-88-9) Tetrahydrofuran(109-99-9) n-hexane(110-54-3) cholest-1-en-3-one Cholest-4-en-3-one (601-57-0) acetoxy Cholesta coprostane(481-21-0) sulfuric acid-methanol 3-acetoxycholest-2-ene
8. S. Yamamura, Chem. Commun., 1494 (1968). 9. M. Toda, M. Hayashi, Y. Hirata, and S. Yamamura, Bull. Chem. Soc. Jpn., 45, 264 (1972). 10. M. I. Elphimoff-Felkin and P. Sarda, Tetrahedron Lett., 3045 (1969). 11. H. O. House, "Modern Synthetic Reactions," W. A. Benjamin, New York, 1965, p. 58; J. G. St. C. Buchanan and P. D. Woodgate, Q, Rev. (London), 23, 522 (1969) and references cited therein. Appendix Chemical Abstracts Nomenclature (Collective Index Number); (Registry Number) ethanol (64-17-5) hydrogen chloride, hydrochloric acid (7647-01-0) Benzene (71-43-2) ether, diethyl ether (60-29-7) acetic anhydride (108-24-7) sodium chloride (7647-14-5) acetone (67-64-1) zinc (7440-66-6) magnesium sulfate (7487-88-9) Cholestanone, cholestan-3-one (566-88-1) Tetrahydrofuran (109-99-9) n-hexane (110-54-3) cholest-1-en-3-one Cholest-4-en-3-one (601-57-0) acetoxy Cholestane, coprostane (481-21-0) sulfuric acid-methanol 3-acetoxycholest-2-ene

cyclopropanol acetate Syntheses,Ine All Rights Reserved
cyclopropanol acetate Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved