正电子在材料科学中的应用 叶邦角 中国科学技术大学理学院 PDF文件使用"pdfFactory”试用版本创建f3 fineprint.cn
正电子在材料科学中的应用 叶邦角 中国科学技术大学理学院 PDF 文件使用 "pdfFactory" 试用版本创建 3www.fineprint.cn ÿf3

第一讲 正电子概况(① 山正电子发现 口正电子的特性 口正电子产生和正电子源 口正电子偶素 PDF文件使用"pdfFactory”试用版本创建ww.fineprint.cn
正电子概况(I) 第一讲 正电子发现 正电子的特性 正电子产生和正电子源 正电子偶素 PDF 文件使用 "pdfFactory" 试用版本创建 ÿwww.fineprint.cn

一、正电子发现 1897年J.J.Thomson 发现电子 1913年E.Rutherford粒子散射 1919年E.Rutherford质子,F.Hess气球实验(宇宙线 1932年Chadwick发现了中子 1931-33年Pauly,Fermi>对原子核的B衰变谱的解释: 中微子(⑤6年实验证实) 1932年Anderson发现反电子(e) 1935年H.Yukawa预言存在π介子,M~1/7M. 1936年Anderson在宇宙线中发现“介子”(u-轻子) PDF文件使用"pdfFactory”试用版本创建ww.fineprint..cn
一、正电子发现 1897年J.J.Thomson 发现电子 1913年E.Rutherford a 粒子散射 1919年E.Rutherford 质子, F.Hess 气球实验 (宇宙线) 1932年Chadwick发现了中子 1931-33年Pauly,Fermi对原子核的 b 衰变谱的解释: 中微子(56年实验证实) 1932年Anderson发现反电子(e+ ) 1935年H.Yukawa 预言存在p介子,Mp~1/7 Mp 1936年Anderson 在宇宙线中发现“介子”( m-轻子) PDF 文件使用 "pdfFactory" 试用版本创建 ÿwww.fineprint.cn
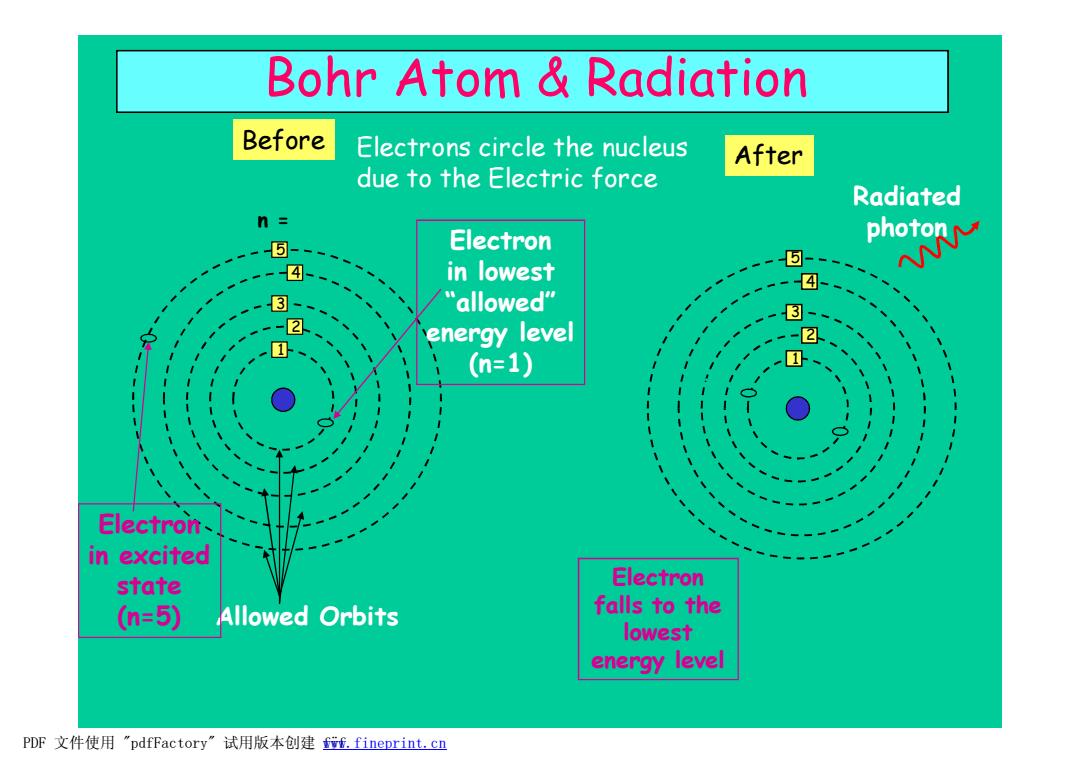
Bohr Atom Radiation Before Electrons circle the nucleus After due to the Electric force Radiated n= ⑤- Electron in lowest ph米 -④ 3- "allowed" 3- energy level (n=1) Electron- in excited state Electron (n=5) Allowed Orbits falls to the lowest energy level PDF文件使用"pdfFactory”试用版本创建ff.fineprint.cn
Electrons circle the nucleus due to the Electric force Bohr Atom & Radiation Allowed Orbits 1 2 3 4 5 n = Electron in lowest “ allowed” energy level (n=1) Electron in excited state (n=5) Before 1 2 3 4 5 Electron falls to the lowest energy level After Radiated photon PDF 文件使用 "pdfFactory" 试用版本创建 f www.fineprint.cn ÿf

Atomic Radiation It is now "known"that when an electron is in an excited state",it spontaneously decays to a ower-energy stable state. The difference in energy,AE,is given by: E5>E4>E3>E2>E One example could be: AE E5-E1=hv=Ephoton Energy Electron Energy Electron h=Planck's constant=6.6x10-34 [J s] in excited in lowest v=frequency of light [hz] state state (higher PE) (lower PE) The energy of the light is DIRECTLY PROPORTIONAL to the frequency,v. n=5 n=5 n=4 E4 Recall that the frequency,v,is related to n=4 the wavelength by: n=3 n=3 c=V入 (V=c/) n=2 E2 n=2 E, So,higher frequency higher energy n=1 n-1 →lower wavelength Before After This is why UV radiation browns your skin but visible light does not PDF文件使用"pdfFactory'”试用版本创建w,fineprint.cn
Atomic Radiation The difference in energy, DE, is given by: DE = E5 – E1 = hn = Ephoton h = Planck’s constant = 6.6x10-34 [J s] n = frequency of light [hz] The energy of the light is DIRECTLY PROPORTIONAL to the frequency, n. Recall that the frequency, n, is related to the wavelength by: c = n l (n = c / l) So, higher frequency è higher energy è lower wavelength This is why UV radiation browns your skin but visible light does not ! It is now “known” that when an electron is in an “ excited state” ,it spontaneously decays to a lower-energy stable state. Before n = 1 n = 2 n = 3 n = 4 n = 5 Energy Electron in excited state (higher PE) E5 E4 E2 E3 E1 E5 > E4 > E3 > E2 > E1 After n = 1 n = 2 n = 3 n = 4 n = 5 Energy Electron in lowest state (lower PE) E5 E4 E2 E3 E1 One example could be: PDF 文件使用 "pdfFactory" 试用版本创建 ÿÿwww.fineprint.cn

Hydrogen atom energy "levels" Quantum physics provides the tools to compute the values of E1,E2,E3,etc...The results are: En=-13.6/n2 -☑ 3- Energy Level Energy E (eV) -2 1 -13.6 2 -3.4 3 -1.51 4 -0.85 5 -0.54 These results DO DEPEND ON THE TYPE OFATOM OR MOLECULE So,the difference in energy between the 3d and 1st quantum state is: Eim=E3-E1=-1.51-(-13.6)=12.09(cV When this 31 atomic transition occurs,this energy is released in the form of electromagnetic energy. PDF文件使用"pdfFactory”试用版本创建www.fineprint.cn
Hydrogen atom energy “levels” Quantum physics provides the tools to compute the values of E1 , E2 , E3 , etc…The results are: En = -13.6 / n2 Energy Level Energy En (eV) 1 -13.6 2 -3.4 3 -1.51 4 -0.85 5 -0.54 So, the difference in energy between the 3rd and 1st quantum state is: Ediff = E3 – E1 = -1.51 – (-13.6) = 12.09 (eV) When this 3à 1 atomic transition occurs, this energy is released in the form of electromagnetic energy. These results DO DEPEND ON THE TYPE OF ATOM OR MOLECULE 1 2 3 4 5 PDF 文件使用 "pdfFactory" 试用版本创建 www.fineprint.cn
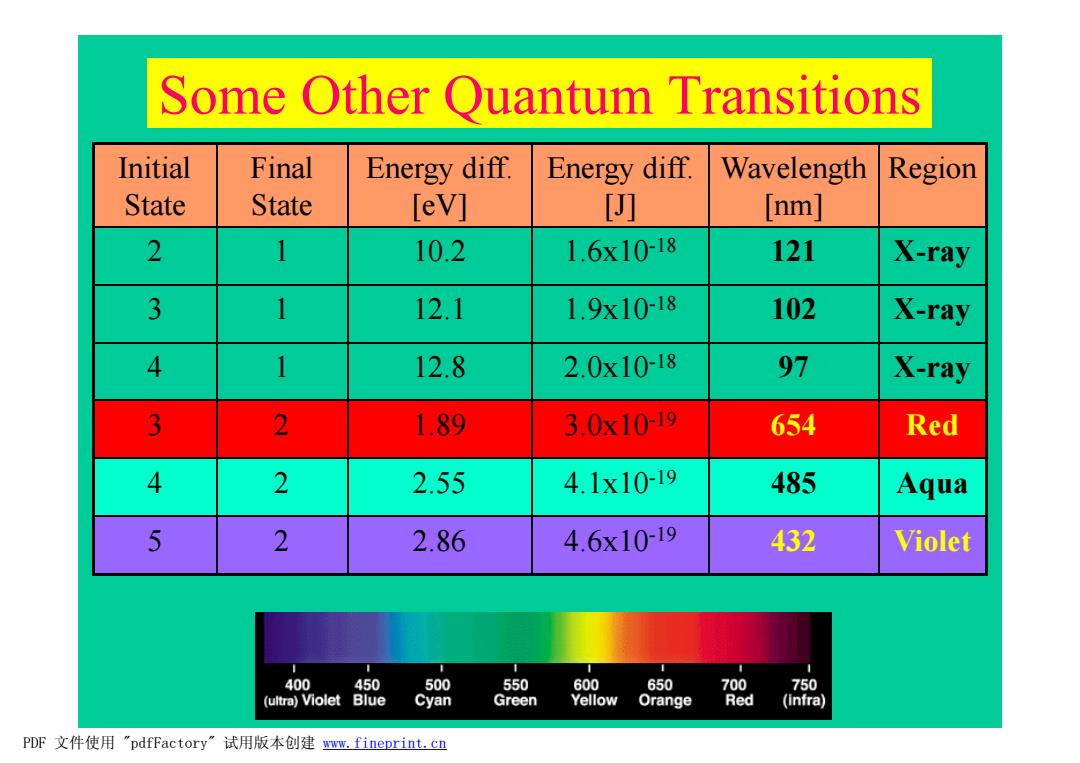
Some Other Quantum Transitions Initial Final Energy diff. Energy diff. Wavelength Region State State [eV] [ [nm] 2 1 10.2 1.6x1018 121 X-ray 3 1 12.1 1.9x1018 102 X-ray 4 1 12.8 2.0x10-18 97 X-ray 3 2 1.89 3.0x10-19 654 Red 4 2 2.55 4.1x10-19 485 Aqua 5 2 2.86 4.6x1019 432 Violet 400 450 500 550 600 650 700 750 (ultra)Violet Blue Cyan Green Yellow Orange Red (infra) PDF文件使用"pdfFactory”试用版本创建www.fineprint.cn
Some Other Quantum Transitions Initial State Final State Energy diff. [eV] Energy diff. [J] Wavelength [nm] Region 2 1 10.2 1.6x10-18 121 X-ray 3 1 12.1 1.9x10-18 102 X-ray 4 1 12.8 2.0x10-18 97 X-ray 3 2 1.89 3.0x10-19 654 Red 4 2 2.55 4.1x10-19 485 Aqua 5 2 2.86 4.6x10-19 432 Violet PDF 文件使用 "pdfFactory" 试用版本创建 www.fineprint.cn

Cosmic rays Cosmic Rays are energetic particles that impinge on our atmosphere. Primary Cosmlc Rays They come from all directions. When these high energy particles strike atoms/molecules in our atmosphere,they produce a spray of particles. Many“exotic”particles can be created.As long as they are not so massive as to violate energy conservation. Some of these particles are unstable and“decay”quickly into other stable particles. Any of these exotic particles can reach the surface of the earth. PDF文件使用"pdfFactory”试用版本创建ww.fineprint.cn
Cosmic Rays q Cosmic Rays are energetic particles that impinge on our atmosphere. q They come from all directions. qWhen these high energy particles strike atoms/molecules in our atmosphere, they produce a spray of particles. q Many “exotic” particles can be created.As long as they are not so massive as to violate energy conservation. q Some of these particles are unstable and “decay” quickly into other stable particles. q Any of these exotic particles can reach the surface of the earth. PDF 文件使用 "pdfFactory" 试用版本创建 ÿwww.fineprint.cn
Discoveries in Cosmic Rays >1932 Discovery of the antiparticle of the electron, the positron.Confirmed the existence and prediction that anti-matter does exist!!! >1937:Discovery of the muon. It's very much like a "heavy electron'”. >1947:Discovery of the pion We'll touch on these today...and some other things... PDF文件使用"pdfFactory”试用版本创建ww.fineprint.cn
Discoveries in Cosmic Rays Ø 1932 : Discovery of the antiparticle of the electron, the positron. Confirmed the existence and prediction that anti-matter does exist!!! Ø 1937 : Discovery of the muon. It’s very much like a “heavy electron” . Ø 1947 : Discovery of the pion. We’ll touch on these today… and some other things… PDF 文件使用 "pdfFactory" 试用版本创建 ÿwww.fineprint.cn
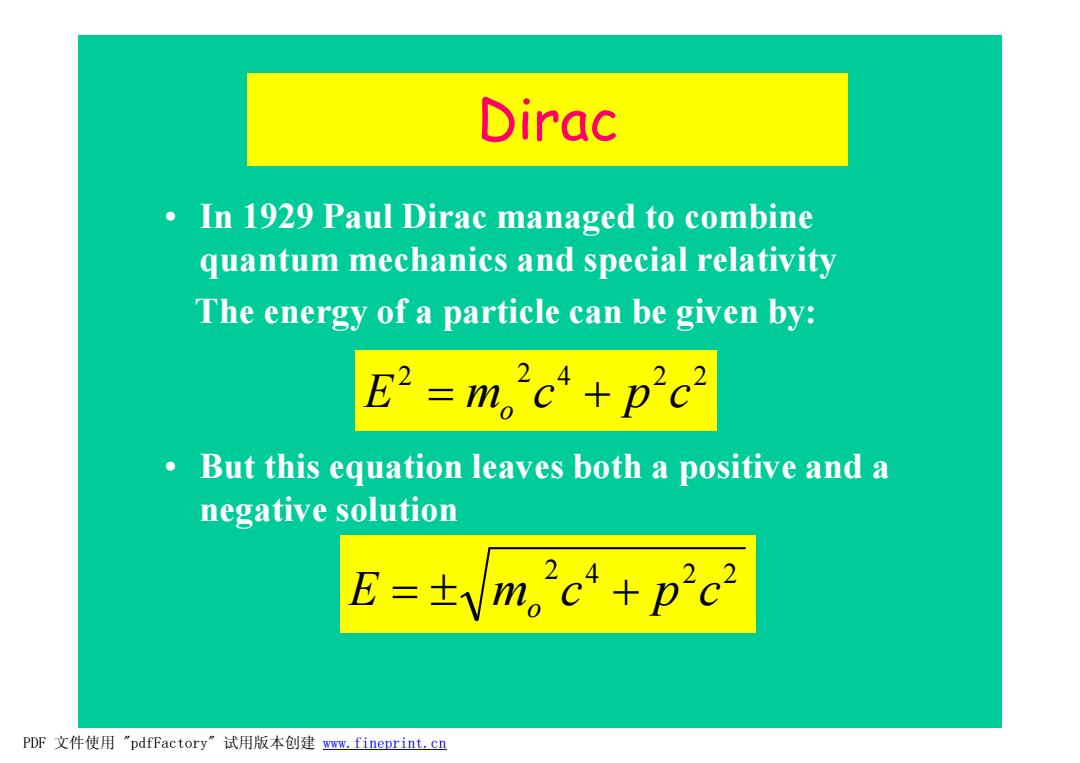
Dirac In 1929 Paul Dirac managed to combine quantum mechanics and special relativity The energy of a particle can be given by: E2=m,2c4+p2c2 But this equation leaves both a positive and a negative solution E=±Vm,2c4+p2c2 PDF文件使用"pdfFactory”试用版本创建www.fineprint.cn
Dirac • In 1929 Paul Dirac managed to combine quantum mechanics and special relativity The energy of a particle can be given by: • But this equation leaves both a positive and a negative solution 2 2 4 2 2 E m c p c = o + 2 4 2 2 E m c p c = ± o + PDF 文件使用 "pdfFactory" 试用版本创建 www.fineprint.cn