
Heat pumping processes and systems Working fluids in Refrigeration and Heat Pump Systems Wednesday July 2nd 2014 Trygve M.Eikevik Professor Norwegian University of Science and Technology(NTNU) E-mails:trygve.m.eikevik@ntnu.no http://folk.ntnu.no/tme 髓 ONTNU ②SINTEF Topics of the lectures General about the refrigerants-history ·Working fluids HFC CFO -R404A 3 HeFC -R407C HC O2 -R410A -R134a -Ammonia -Hydrocarbons (example.propane) -Carbon dioxide(CO2) Choice of working fluid Refrigeration process with different working fluids Practical qualities for different working fluids ONTNU 2 ⑤SINTEF
1 Heat pumping processes and systems Working fluids in Refrigeration and Heat Pump Systems Wednesday July 2nd 2014 Trygve M. Eikevik Professor Norwegian University of Science and Technology (NTNU) E-mails: trygve.m.eikevik@ntnu.no http://folk.ntnu.no/tme 2 Topics of the lectures • General about the refrigerants - history • Working fluids – R404A – R407C – R410A – R134a – Ammonia – Hydrocarbons (example. propane) – Carbon dioxide (CO2 ) • Choice of working fluid • Refrigeration process with different working fluids • Practical qualities for different working fluids HFC NH3 HC CO2 CFC HCFC

Working fluid,refrigerant,process fluid Working fluid transports energy/heat from cold to warm side in the refrigeration system and through phase change between liquid and gas in a closed circle process(vapor compression process) Important factors on choice of working fluid -Temperature-/operation range -System solutions 一Place of operation -Availability and price -Locale environmental consequences(safety) -Global environmental consequences ONTNU ②SINTEF Working fluids -development Before 1930-"Natural fluids",example sulphuric ether,SO2,methyl chloride. ammonia,propane,isobutene etc. 1930/50-Synthetic fluids,example CFC-12 and HCFC-22 1987-Montreal protocol established.CFC and HCFC ozon destructive due to chloride and bromine.Phase out of CFC(1995)and HCFC(2010) .1988-HydrogenFluorcarbons introduced(HFC) .1997-Kyoto protocol established.HFC regulations due to high GWP-values. CO2-tax on HFc from 2003(the tax is depending in the GWP-value). 1990-now-Increased focus on use of NATURAL WORKING FLUIDS. special ammonia,CO,and hydro carbons 回NTNU SINTEF
3 Working fluid, refrigerant, process fluid • Working fluid transports energy/heat from cold to warm side in the refrigeration system and through phase change between liquid and gas in a closed circle process (vapor compression process) • Important factors on choice of working fluid – Temperature-/operation range – System solutions – Place of operation – Availability and price – Locale environmental consequences (safety) – Global environmental consequences 4 Working fluids – development • Before 1930 – ”Natural fluids”, example sulphuric ether , SO2 , methyl chloride, ammonia, propane, isobutene etc. • 1930/50 – Synthetic fluids, example CFC-12 and HCFC-22 • 1987 – Montreal protocol established . CFC and HCFC ozon destructive due to chloride and bromine. Phase out of CFC (1995) and HCFC (2010) • 1988 – HydrogenFluorcarbons introduced (HFC) • 1997 – Kyoto protocol established. HFC regulations due to high GWP-values. CO2 -tax on HFc from 2003 (the tax is depending in the GWP-value). • 1990 - now – Increased focus on use of NATURAL WORKING FLUIDS, special ammonia, CO2 and hydro carbons

Working fluids-important factors Physical-thermodynamic qualities -Normal boiling point(at 1,013 bar) -Vapor pressure curve-critical temperature(Tc)and pressure(Pc): Refrigeration process-process losses .Pressure level-pressure rating of components(21-23/38 bars operation pressure) .Pressure ratio-efficiencies of the compressor -Molecule weight: Heat of evaporation-mg pipe diameters Temperature in the exit of compressor -Volumetric refrigeration capacity-Ah.gas density [kJ/m3] .Gives necessary compressor volume [m] Thermo physical properties -Specific heat capacity,co [kJ/(kgK)] -Viscosity,v[mm2/s] -Thermal conductivity,[W/(mK)] ONTNU ②SINTEF Working fluids-important factors cont. 。Other factors -Chemical stability -Material compatibility(corrosion,reactions) 一Vater solubility -Miscibility with different types of oils/lubricants -Electric resistance Price and availability Environmental friendliness-safety Impact on ozone layer(ODP-Ozone Depleting Potential) -Contribution to the greenhouse effect(GWP-Globa/Warm -Toxicity,inflammability,odor etc. 回NTNU SINTEF
5 Working fluids – important factors • Physical – thermodynamic qualities – Normal boiling point (at 1,013 bar) – Vapor pressure curve – critical temperature (TC) and pressure (PC): • Refrigeration process – process losses • Pressure level – pressure rating of components (21-23/38 bars operation pressure) • Pressure ratio – efficiencies of the compressor – Molecule weight: • Heat of evaporation – mR , pipe diameters • Temperature in the exit of compressor – Volumetric refrigeration capacity – Dh • gas density [kJ/m3 ] • Gives necessary compressor volume [m3 ] • Thermo physical properties – Specific heat capacity, cp [kJ/(kgK)] – Viscosity, n [mm2 /s] – Thermal conductivity, l [W/(mK)] 6 • Other factors – Chemical stability – Material compatibility (corrosion, reactions) – Water solubility – Miscibility with different types of oils / lubricants – Electric resistance • Price and availability • Environmental friendliness - safety – Impact on ozone layer (ODP - Ozone Depleting Potential) – Contribution to the greenhouse effect (GWP - Global Warming Potential) – Toxicity, inflammability, odor etc. Working fluids – important factors cont
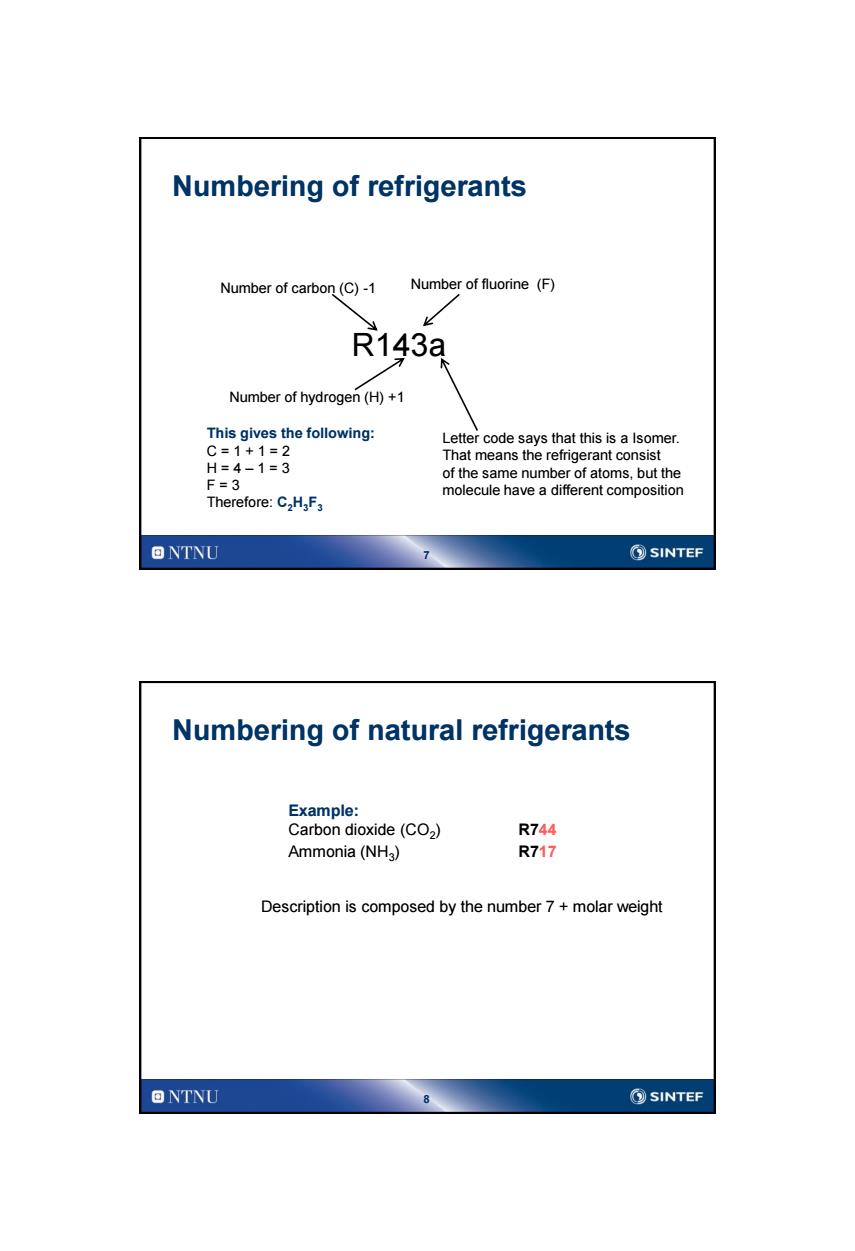
Numbering of refrigerants Number of carbon(C)-1 Number of fluorine (F) R143a Number of hydrogen(H)+1 This gives the following: Letter code says that this is a Isomer. C=1+1=2 That means the refrigerant consist H=4-1=3 of the same number of atoms,but the F=3 molecule have a different composition Therefore:C2H,F3 ONTNU ②SINTEF Numbering of natural refrigerants Example: Carbon dioxide(CO2) R744 Ammonia(NHg) R717 Description is composed by the number 7 molar weight 回NTNU SINTEF
7 Numbering of refrigerants R143a Number of carbon (C) -1 Number of fluorine (F) Number of hydrogen (H) +1 This gives the following: C = 1 + 1 = 2 H = 4 – 1 = 3 F = 3 Therefore: C2H3F3 Letter code says that this is a Isomer. That means the refrigerant consist of the same number of atoms, but the molecule have a different composition 8 Numbering of natural refrigerants Example: Carbon dioxide (CO2 ) R744 Ammonia (NH3 ) R717 Description is composed by the number 7 + molar weight
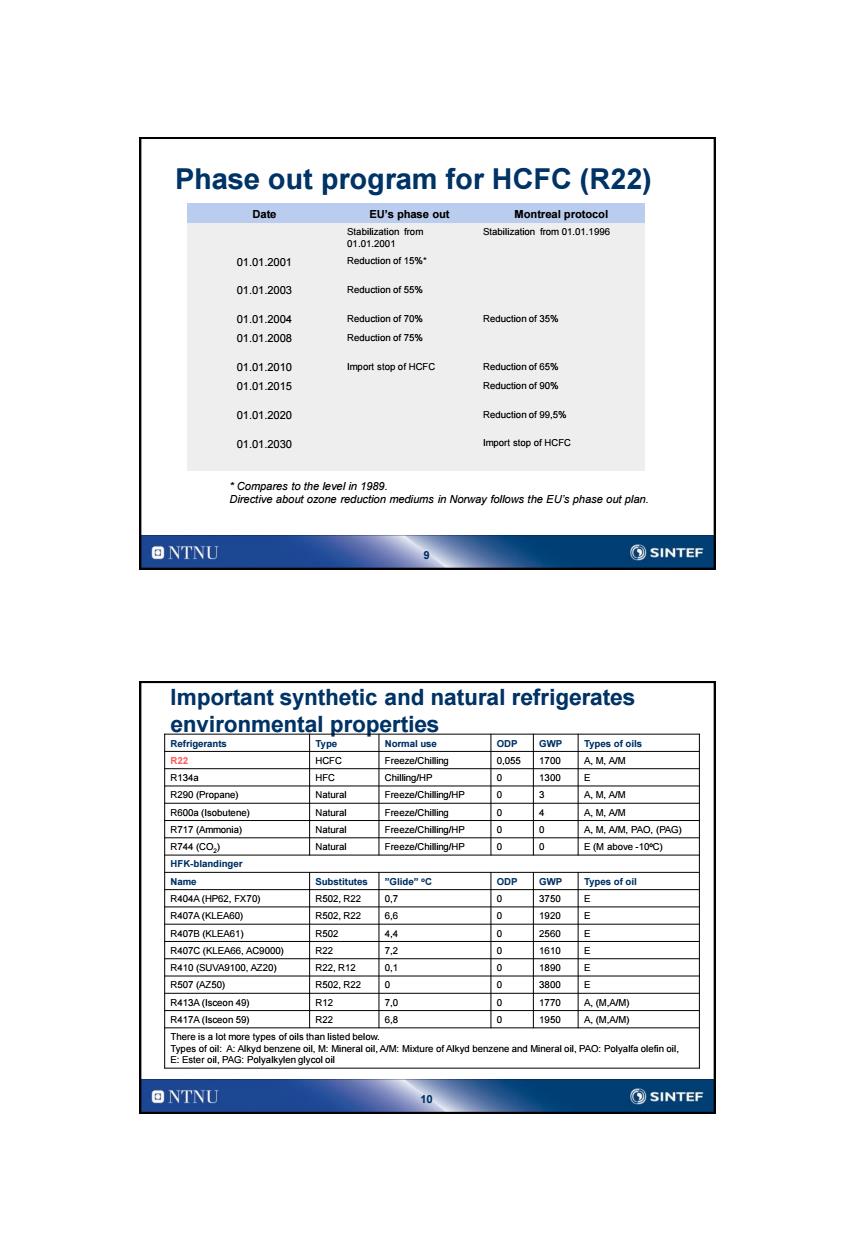
Phase out program for HCFC(R22) Date EU's phase out Montreal protocol Stabilization from Stabilization from 01.01.1996 01.01.2001 01.012001 Reduction of 15%* 01.012003 Reduction of 55% 01.012004 Reduction of 70% Reduction of 35% 01.012008 Reduction of 75% 01.01.2010 Import stop of HCFC Reduction of 65% 01.012015 Reduction of 90% 01.01.2020 Reduction of 99.5% 01.012030 Import stop of HCFC *Compares to the level in 1989. Directive about ozone reduction mediums in Norway follows the EU's phase out plan. ONTNU ②SINTEF Important synthetic and natural refrigerates environmental properties Refrigerants Type Normal use ODP GWP Types of oils R22 HCFC Freeze/Chilling 0.055 1700 A.M.A/M R134a HFC Chilling/HP 0 1300 E R290(Propane) Natural Freeze/Chiling/HP 0 3 A.M.A/M R6ODa(lsobutene) Natural Freeze/Chilling 0 4 A.M.A/M R717 (Ammonia) Natural Freeze/Chilling/HP 0 0 A.M.A/M.PAO.(PAG) R744(C0) Natural Freeze/Chilling/HP 0 0 E(M above-10℃) HFK-blandinger Name Substitutes "Glide"C ODP GWP Types of oil R404AHP62,FX70) R502.R22 07 0 3750E R407A(KLEA60) R502.R22 6,6 0 1920 E R407B(KLEA61) R502 4.4 0 2560 E R407C(KLEA66,AC9000) R22 7.2 0 1610E R410(SUVA9100.AZ20) R22,R12 0.1 0 1890 E R507(AZ50 R502.R22 0 0 3800 E R413A(Isceon 49) R12 7.0 0 1770 A.(M.A/M) R417A(lsceon 59) R22 6,8 0 1950 A.(M.A/M) There is a lot more types of oils than listed below. Types of oil:A:Alkyd benzene oil,M:Mineral oil,A/M:Mixture of Alkyd benzene and Mineral oil,PAO:Polyalfa olefin oil, E:Ester oil,PAG:Polyalkylen glycol oil ONTNU 10 SINTEF
9 Phase out program for HCFC (R22) Date EU’s phase out Montreal protocol Stabilization from 01.01.2001 Stabilization from 01.01.1996 01.01.2001 Reduction of 15%* 01.01.2003 Reduction of 55% 01.01.2004 Reduction of 70% Reduction of 35% 01.01.2008 Reduction of 75% 01.01.2010 Import stop of HCFC Reduction of 65% 01.01.2015 Reduction of 90% 01.01.2020 Reduction of 99,5% 01.01.2030 Import stop of HCFC * Compares to the level in 1989. Directive about ozone reduction mediums in Norway follows the EU’s phase out plan. 10 Important synthetic and natural refrigerates environmental properties Refrigerants Type Normal use ODP GWP Types of oils R22 HCFC Freeze/Chilling 0,055 1700 A, M, A/M R134a HFC Chilling/HP 0 1300 E R290 (Propane) Natural Freeze/Chilling/HP 0 3 A, M, A/M R600a (Isobutene) Natural Freeze/Chilling 0 4 A, M, A/M R717 (Ammonia) Natural Freeze/Chilling/HP 0 0 A, M, A/M, PAO, (PAG) R744 (CO2 ) Natural Freeze/Chilling/HP 0 0 E (M above -10oC) HFK-blandinger Name Substitutes ”Glide” oC ODP GWP Types of oil R404A (HP62, FX70) R502, R22 0,7 0 3750 E R407A (KLEA60) R502, R22 6,6 0 1920 E R407B (KLEA61) R502 4,4 0 2560 E R407C (KLEA66, AC9000) R22 7,2 0 1610 E R410 (SUVA9100, AZ20) R22, R12 0,1 0 1890 E R507 (AZ50) R502, R22 0 0 3800 E R413A (Isceon 49) R12 7,0 0 1770 A, (M,A/M) R417A (Isceon 59) R22 6,8 0 1950 A, (M,A/M) There is a lot more types of oils than listed below. Types of oil: A: Alkyd benzene oil, M: Mineral oil, A/M: Mixture of Alkyd benzene and Mineral oil, PAO: Polyalfa olefin oil, E: Ester oil, PAG: Polyalkylen glycol oil
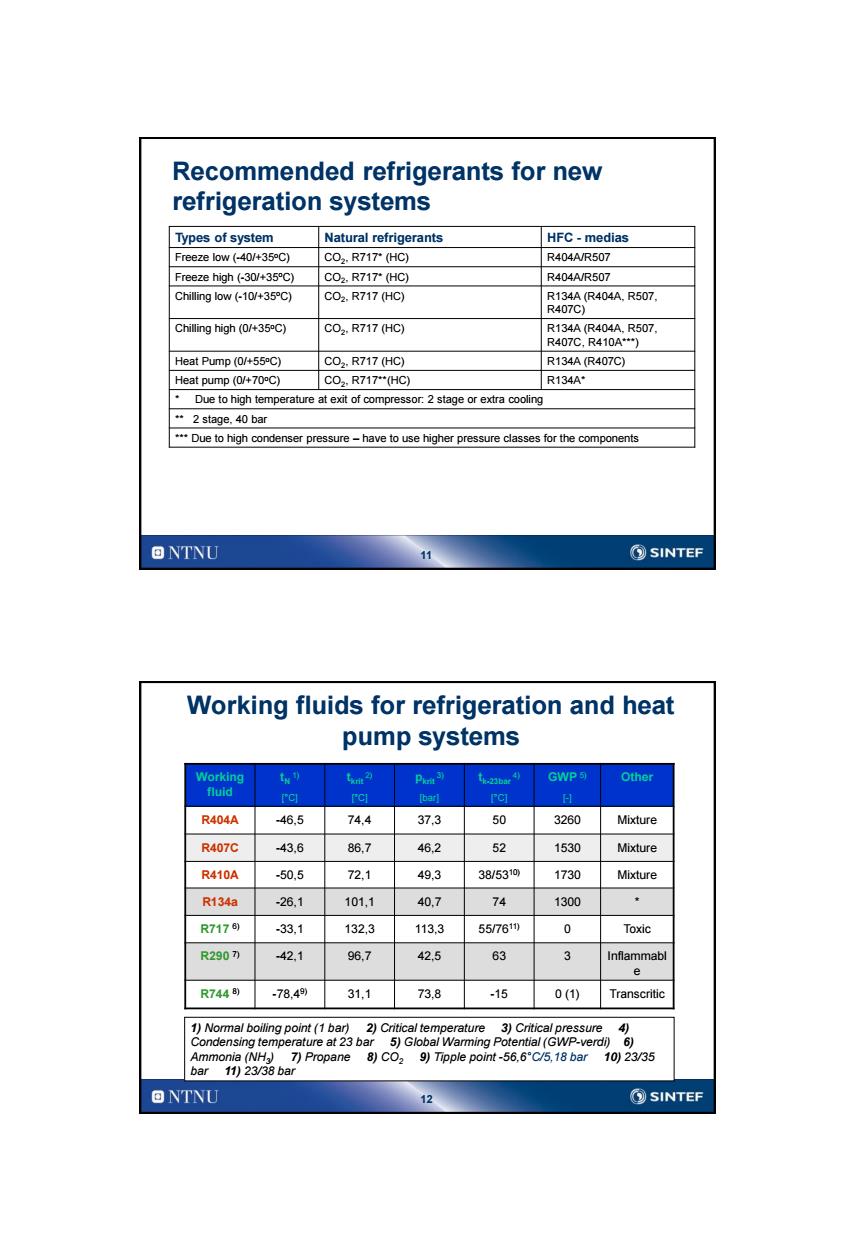
Recommended refrigerants for new refrigeration systems Types of system Natural refrigerants HFC-medias Freeze low (-40/+35C) C02.R717T(HC) R404A/R507 Freeze high(-30/+35C) C02,R717(HC) R404A/R507 Chilling low (-10/+35C) C02,R717(HC) R134A(R404A.R507, R407C) Chilling high(0/+35C) C02.R717(HC) R134A(R404A.R507. R407C,R410A*) Heat Pump (0/+55C) C02.R717(HC) R134A(R407C) Heat pump(0/+70C) C02,R717(HC) R134A Due to high temperature at exit of compressor:2 stage or extra cooling “2 stage,.40bar Due to high condenser pressure-have to use higher pressure classes for the components ONTNU 11 ②SINTEF Working fluids for refrigeration and heat pump systems Working tx) txm2 Piors3) t.23bae4) GWP5 Other fluid rcl C [barl PCI 日H R404A 46.5 74,4 373 50 3260 Mixture R407C -43.6 86,7 46.2 52 1530 Mixture R410A -50.5 72,1 49.3 38/5310 1730 Mixture R134a -26.1 101,1 40.7 74 1300 R7176) -33,1 132,3 113,3 55/761 0 Toxic R290) -42.1 96.7 42,5 63 3 Inflammabl e R7448) -78.49) 31,1 73.8 -15 0(1) Transcritic 1)Normal boiling point(1 bar)2)Critical temperature 3)Critical pressure 4) Condensing temperature at 23 bar 5)Global Warming Potential(GWP-verdi)6) Ammonia(NH 7)Propane 8))Tipple point-56.6"C/5,18 bar 10)23/35 bar 11)23/38 bar ONTNU SINTEF
11 Recommended refrigerants for new refrigeration systems Types of system Natural refrigerants HFC - medias Freeze low (-40/+35oC) CO2 , R717* (HC) R404A/R507 Freeze high (-30/+35oC) CO2 , R717* (HC) R404A/R507 Chilling low (-10/+35oC) CO2 , R717 (HC) R134A (R404A, R507, R407C) Chilling high (0/+35oC) CO2 , R717 (HC) R134A (R404A, R507, R407C, R410A***) Heat Pump (0/+55oC) CO2 , R717 (HC) R134A (R407C) Heat pump (0/+70oC) CO2 , R717**(HC) R134A* * Due to high temperature at exit of compressor: 2 stage or extra cooling ** 2 stage, 40 bar *** Due to high condenser pressure – have to use higher pressure classes for the components 12 Working fluids for refrigeration and heat pump systems Working fluid tN 1) [°C] tkrit 2) [°C] pkrit 3) [bar] tk-23bar 4) [°C] GWP 5) [-] Other R404A -46,5 74,4 37,3 50 3260 Mixture R407C -43,6 86,7 46,2 52 1530 Mixture R410A -50,5 72,1 49,3 38/5310) 1730 Mixture R134a -26,1 101,1 40,7 74 1300 * R717 6) -33,1 132,3 113,3 55/7611) 0 Toxic R290 7) -42,1 96,7 42,5 63 3 Inflammabl e R744 8) -78,49) 31,1 73,8 -15 0 (1) Transcritic 1) Normal boiling point (1 bar) 2) Critical temperature 3) Critical pressure 4) Condensing temperature at 23 bar 5) Global Warming Potential (GWP-verdi) 6) Ammonia (NH3 ) 7) Propane 8) CO2 9) Tipple point -56,6°C/5,18 bar 10) 23/35 bar 11) 23/38 bar
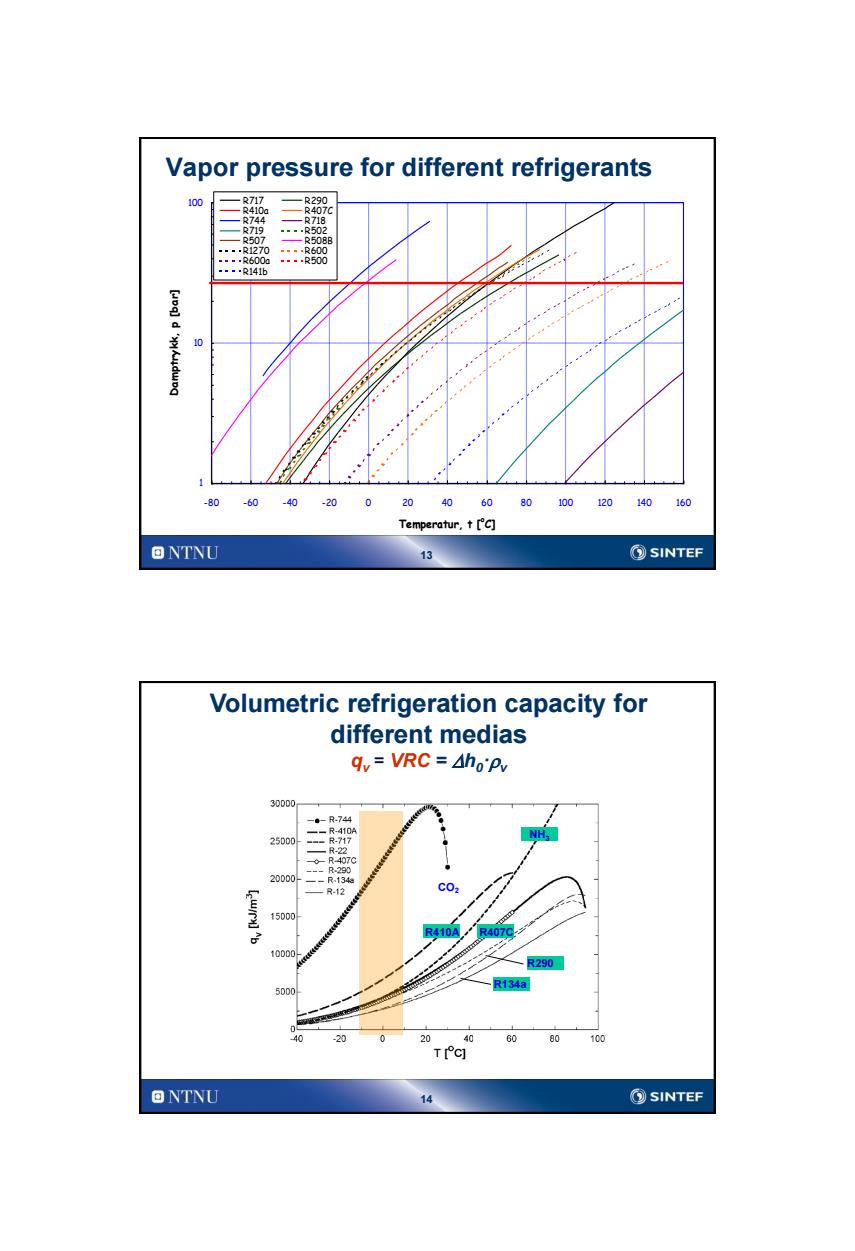
Vapor pressure for different refrigerants 100 .R717 R290 R407C ,R744 R718 R719 ---.R502 R507 R508B .-.R1270-….…R600 ----R600a---.R500 ▣▣▣.R141b 10 -80 -60 .40 20 60 80 100 120 140 160 Temperatur,t['c] ONTNU SINTEF Volumetric refrigeration capacity for different medias qy=VRC=Aho'Pv 30000 -。-R-744 -R-410A NH 25D00 R-22 R-407C 20D00 -R-12 C02 15000 R410A/ R407 10D0D0 R290 R134a 5000 0 20 0 60 80 100 TIC] ONTNU ⑤SINTEF
13 Vapor pressure for different refrigerants 1 10 100 -80 -60 -40 -20 0 20 40 60 80 100 120 140 160 Temperatur, t [oC] Damptrykk, p [bar] R717 R290 R410a R407C R744 R718 R719 R502 R507 R508B R1270 R600 R600a R500 R141b 14 Volumetric refrigeration capacity for different medias qv = VRC = Dh0·v CO2 R134a R407C R290 R410A NH3

Exit temperature of compressor for different fluids Variable to,t=40C,n=70% 9 300 =40℃ 6=0,7 250 200 150 月41A 100 HFC-1344 R-50TA- 40 30 20 10 10 Saturation temperature,t(C) .HFC fluids(POE),propane(MO,POE)-maximum exit temperature of the compressor 120c Ammonia(PAO)-maximum exit temperature of the compressor 160C ONTNU ②SINTEF Pressure levels Curves for vapor pressure as function of the saturation temperature 0 R744 Operation pressure -R-4TO in CO,-systems is ==R-717 typical 5 to 10 times 。-R-4070 73,8 bar higher than with use 民22-- R280 of HFC and ---R134h ammonia -R12 C02 Normal refrigeration R22 and heat pump R410A R290 systems are limited R134a to approx 28C 25bar R407C condensing R12 temperature Necessary with actions to handle 40 20 0 20 40 60 0 100 high deadhead Saturation temperature,t [C] pressure during longer stops ONTNU 16 SINTEF
15 Exit temperature of compressor for different fluids Variable t0 , tc=40°C, hi=70% • HFC fluids (POE), propane (MO, POE) – maximum exit temperature of the compressor 120ºC • Ammonia (PAO) – maximum exit temperature of the compressor 160ºC Saturation temperature, to ( oC) Compressor discharge temperature, oC 16 Pressure levels Curves for vapor pressure as function of the saturation temperature • Operation pressure in CO2 -systems is typical 5 to 10 times higher than with use of HFC and ammonia • Normal refrigeration and heat pump systems are limited to approx 28°C condensing temperature • Necessary with actions to handle high deadhead pressure during longer stops CO2 NH3 R134a R407C R410A R290 R12 R22 25 bar 73,8 bar Metningstemperatur [°C] Pressure [MPa] Saturation temperature, t [oC]

Temperature losses versus pressure losses Progress of pressure curve-At/Ap [K/Pa] 0.2 。Loss in saturation 一R.12 temperature due to -R13 given pressure loss 0.15 -R290 -R-407C (tU△pj)for CO2is5to 一-R22 10 times lower than dyAv R407C R-717 HFC and ammonia --R41M 0.1 --R-744 Dimensioning of heat exchangers with R410A R134a relative high pressure. Gives high heat 0.05 R12 transfer. CO2 Pipes can be designed N R290 for high velocities -20 2n 100 without having high Saturation temperature,t[C] temperature loss ONTNU 17 ②SINTEF Mixtures of working fluids with HFC Binary or tertiary mixtures for use in heat pump systems ·R404A -HFC-125(44%),HFC-143a(52%),HFC-134a(4%) -Temperature glide approx 0.7 K(approximately azec ·R407C -HFC-125(25%),HFC-32(23%),HFC-134a(52%) -Temperature glide approx.7.5 K(zeotrope) ·R410A -HFC-125(50%),HFC-32(50%) -Temperature glide approx.0.2 K(approximately azeotrop) 回NTNU 18 SINTEF
17 Temperature losses versus pressure losses Progress of pressure curve – Dt/Dp [K/Pa] • Loss in saturation temperature due to given pressure loss (Dt/Dp) for CO2 is 5 to 10 times lower than HFC and ammonia • Dimensioning of heat exchangers with relative high pressure. Gives high heat transfer. • Pipes can be designed for high velocities without having high temperature loss CO2 NH3 R12 R290 Metningstemperatur [°C] Dt/Dp [k/kPa] Saturation temperature, t [oC] 18 Mixtures of working fluids with HFC Binary or tertiary mixtures for use in heat pump systems • R404A – HFC-125 (44%), HFC-143a (52%), HFC-134a (4%) – Temperature glide approx 0.7 K (approximately azeotrope) • R407C – HFC-125 (25%), HFC-32 (23%), HFC-134a (52%) – Temperature glide approx. 7.5 K (zeotrope) • R410A – HFC-125 (50%), HFC-32 (50%) – Temperature glide approx. 0.2 K (approximately azeotrop)
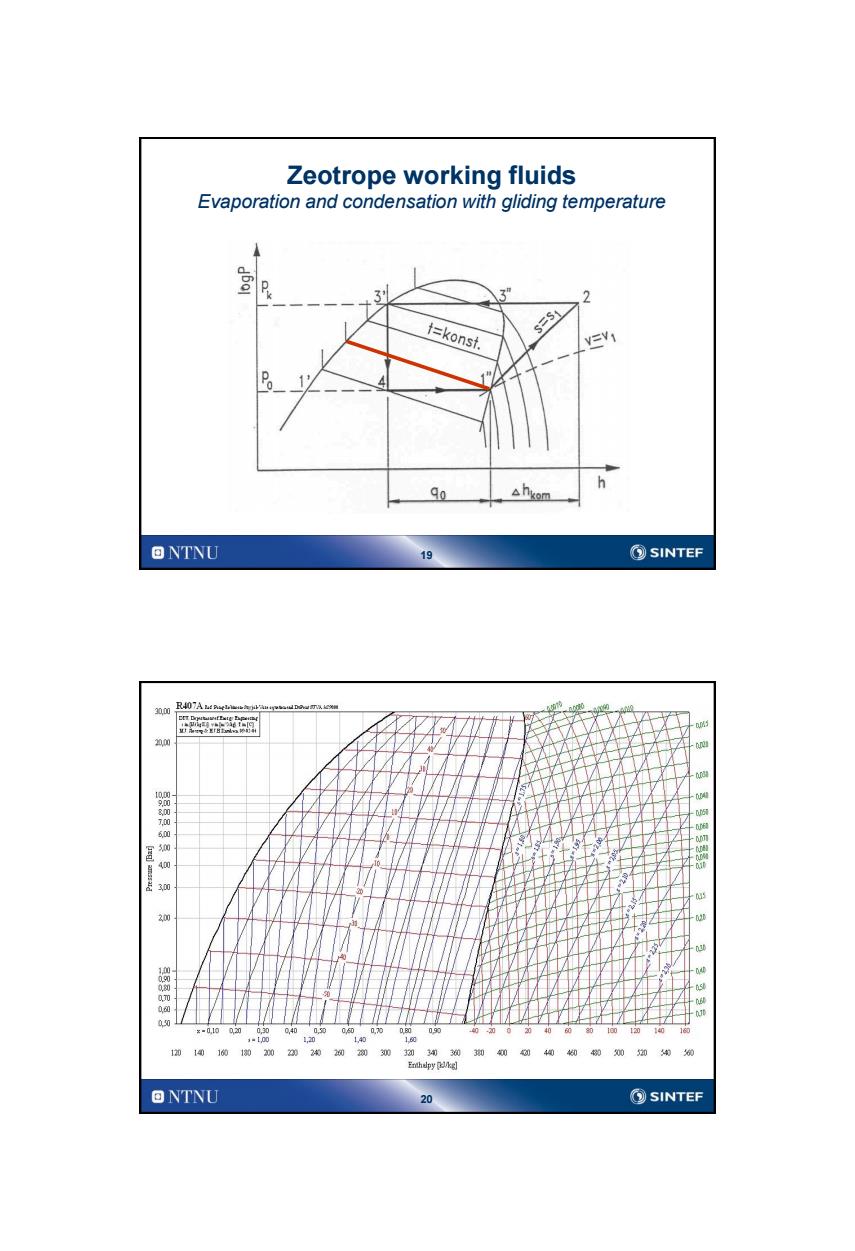
Zeotrope working fluids Evaporation and condensation with gliding temperature 3 3” 2 t=konst s=S1 A hkom h 90 ONTNU 19 SINTEF R407A4a 20.00 30 00 x-0,10 00 00 1 140 10 120 14016010、2002020202033030 340404糊0020 0 Enthelpy lkg] ONTNU 20 ⑤SINTEF
19 Zeotrope working fluids Evaporation and condensation with gliding temperature 20