
Introduction to Chromatographic Separations (Chapter 26) Many determinations involve separation followed by analysis ·chromatography ·electrophoresis Chromatography: sample transported by mobile phase electrostatic or van der Waals' some components in sample interact more strongly with stationary phase and are more strongly retained sample separated into zones or bands CEM 333 page 14.1
Introduction to Chromatographic Separations (Chapter 26) Many determinations involve separation followed by analysis • chromatography • electrophoresis Chromatography: sample transported by mobile phase electrostatic or van der Waals' some components in sample interact more strongly with stationary phase and are more strongly retained sample separated into zones or bands CEM 333 page 14.1
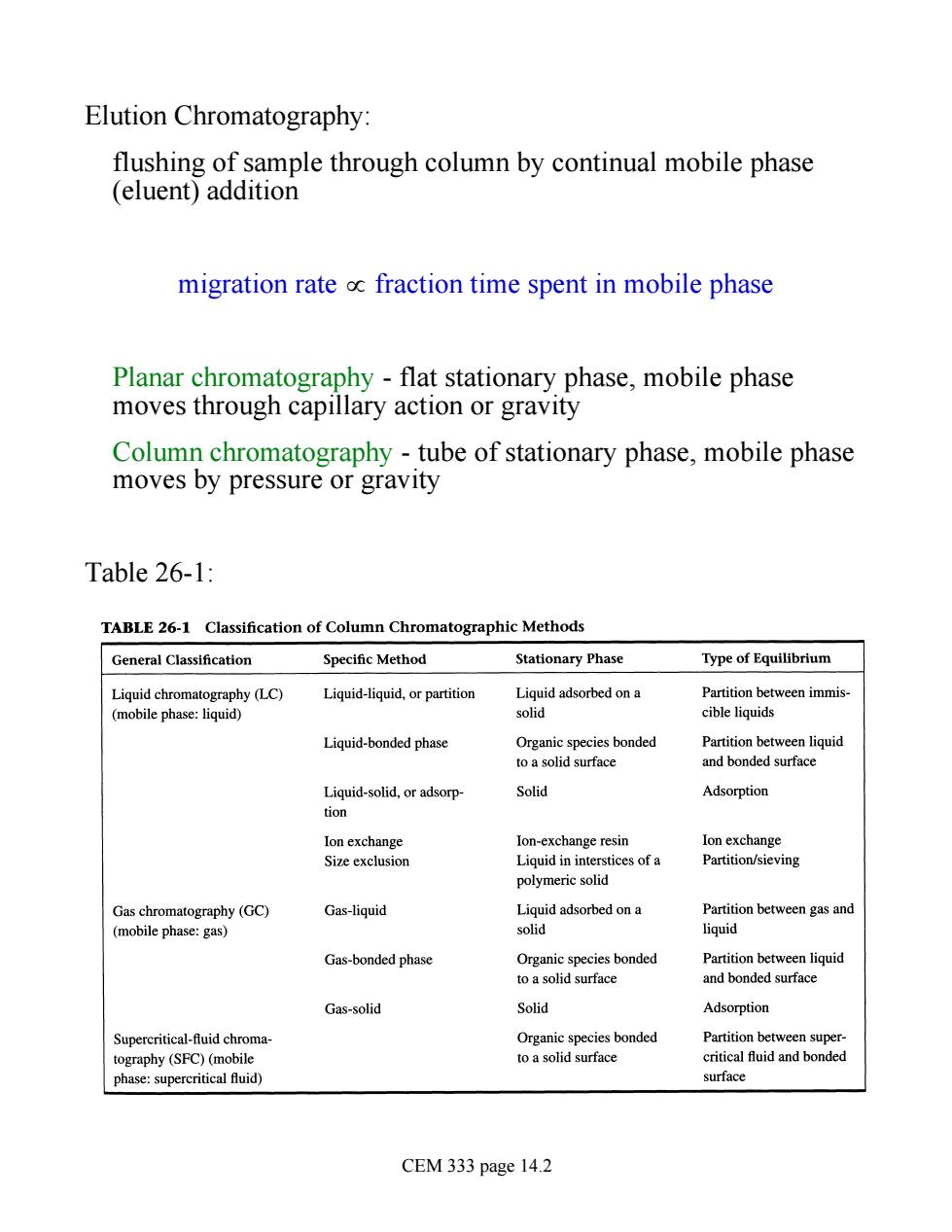
Elution Chromatography: flushing of sample through column by continual mobile phase (eluent)addition migration rate oc fraction time spent in mobile phase Planar chromatography -flat stationary phase,mobile phase moves through capillary action or gravity Column chromatography-tube of stationary phase,mobile phase moves by pressure or gravity Table 26-1: TABLE 26-1 Classification of Column Chromatographic Methods General Classification Specific Method Stationary Phase Type of Equilibrium Liguid-liquid,or partition Liquid adsorbed on a Partition between immis- solid cible liquids Liquid-bonded phase Organic species bonded to a solid surface and bonded surface Solid Adsorption Ion-exchange resin Ion exchange Liquid ininterstices ofa Partition/sieving polymeric solid Gas chromatography(GC) Gas-liquid Liquidadsoredona Partition between gas and (mobile phase:gas) solid liquid Gas-bonded phase Partition bet Gas-solid Solid Adsorption Supercritical-fluid chroma- Organic species bonded Partition between super- tography (SFC)(mobile toa solid surface critical fuid and bonded phase:supercritical fluid) surface CEM 333 page 14.2
Elution Chromatography: flushing of sample through column by continual mobile phase (eluent) addition migration rate µ fraction time spent in mobile phase Planar chromatography - flat stationary phase, mobile phase moves through capillary action or gravity Column chromatography - tube of stationary phase, mobile phase moves by pressure or gravity Table 26-1: CEM 333 page 14.2
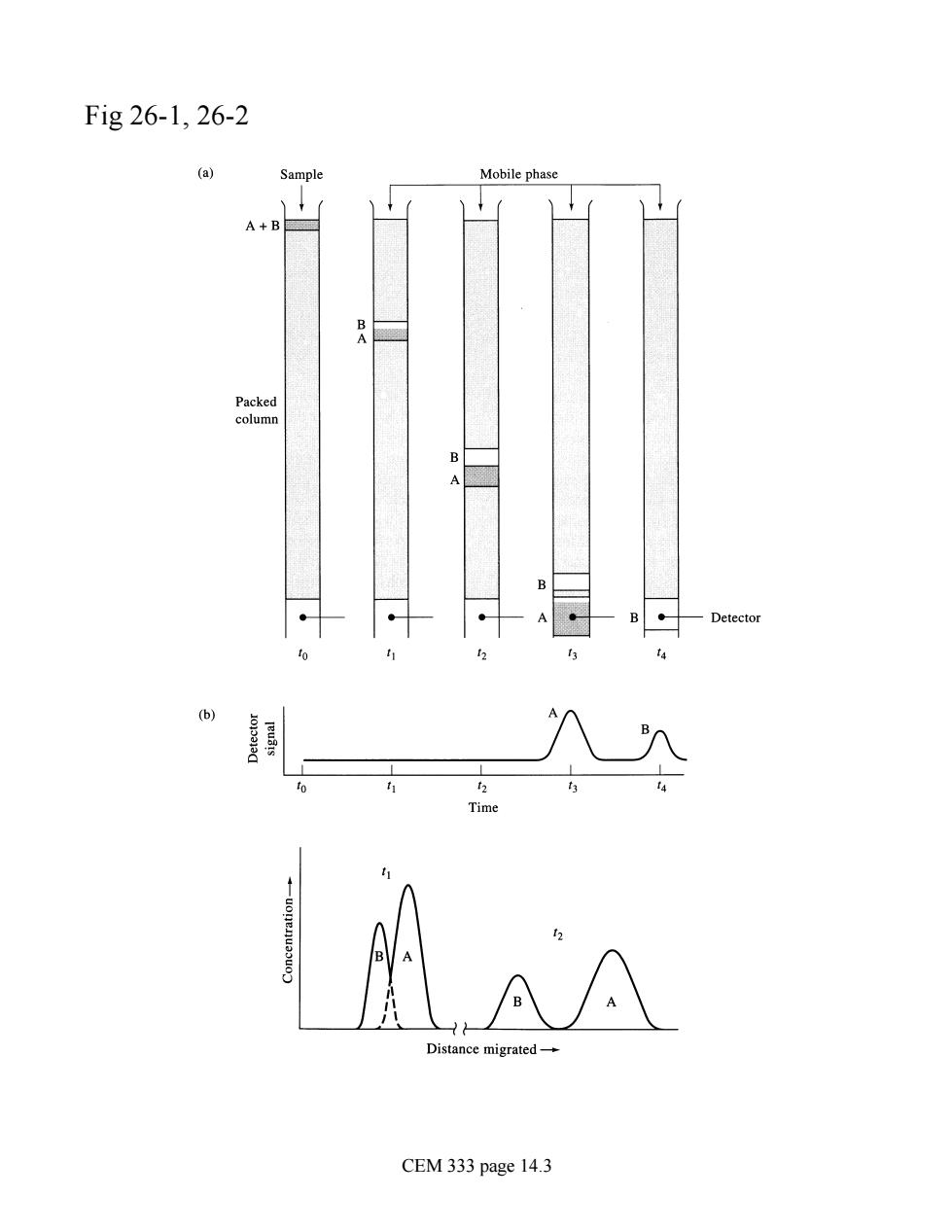
Fig26-1,26-2 Sample Mobile phase tad e migrated CEM 333 page 14.3
Fig 26-1, 26-2 CEM 333 page 14.3
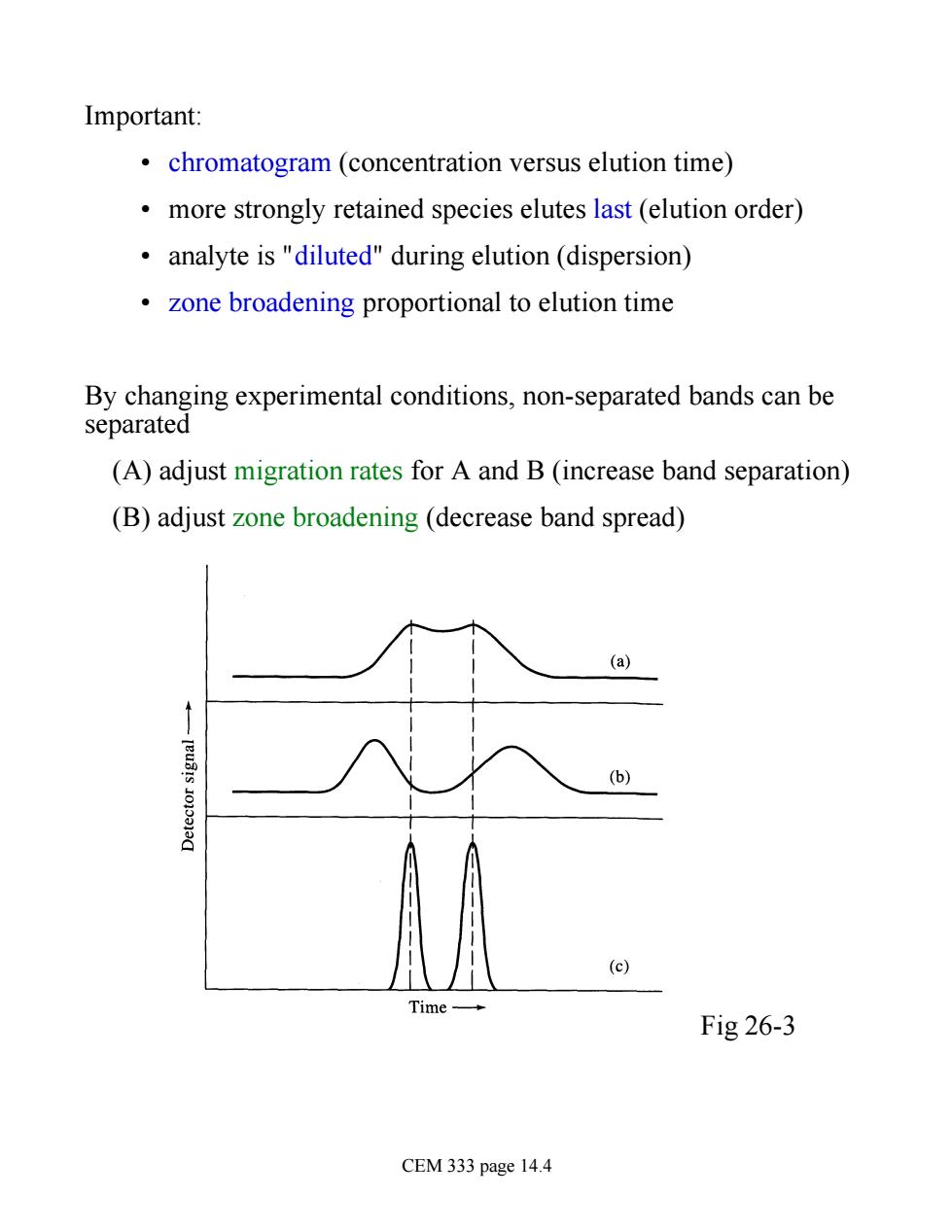
Important: chromatogram (concentration versus elution time) more strongly retained species elutes last (elution order) analyte is "diluted"during elution (dispersion) zone broadening proportional to elution time By changing experimental conditions,non-separated bands can be separated (A)adjust migration rates for A and B(increase band separation) (B)adjust zone broadening(decrease band spread) (a) (b) (c) Time Fig 26-3 CEM 333 page 14.4
Important: • chromatogram (concentration versus elution time) • more strongly retained species elutes last (elution order) • analyte is "diluted" during elution (dispersion) • zone broadening proportional to elution time By changing experimental conditions, non-separated bands can be separated (A) adjust migration rates for A and B (increase band separation) (B) adjust zone broadening (decrease band spread) Fig 26-3 CEM 333 page 14.4
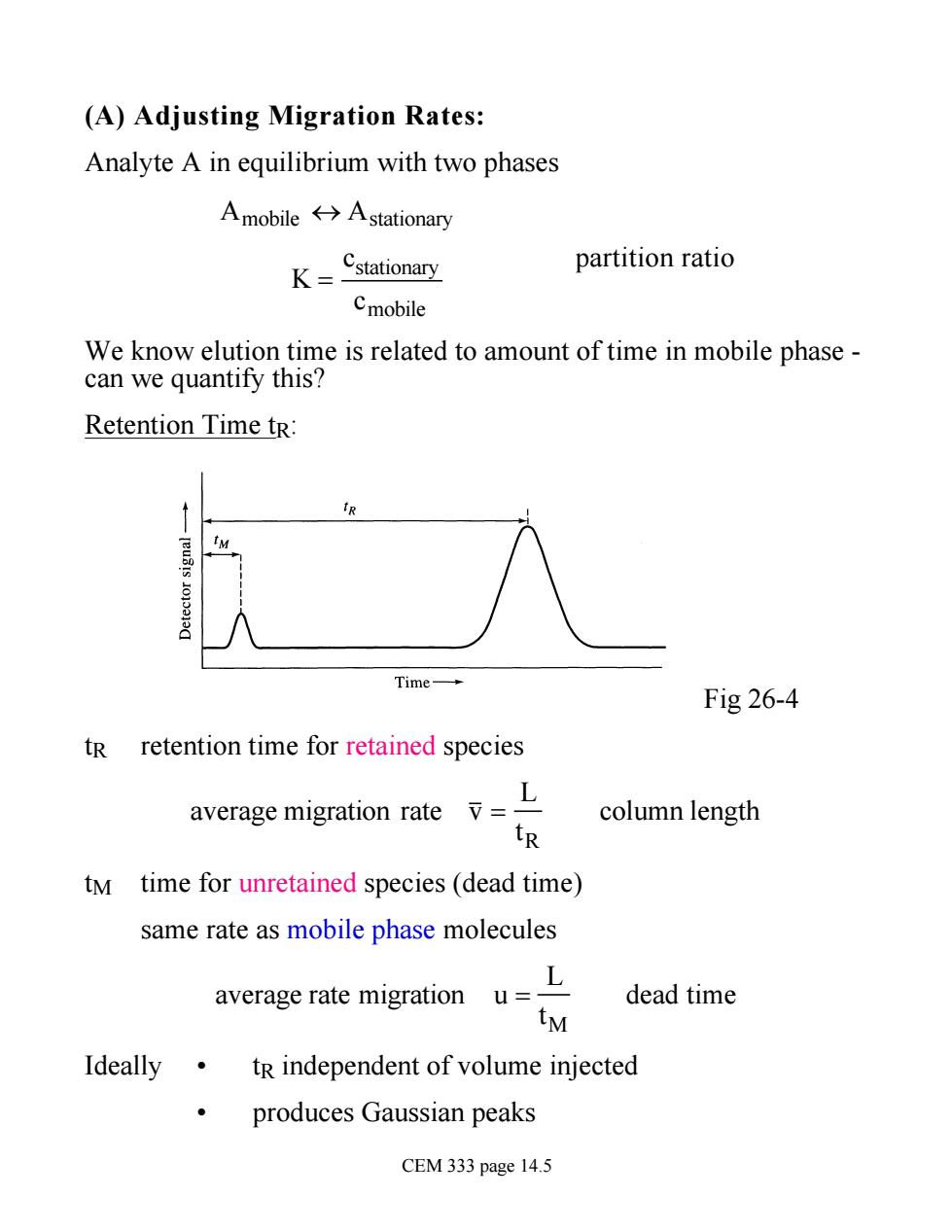
(A)Adjusting Migration Rates: Analyte A in equilibrium with two phases Amobile分Astationary K=Cstationary partition ratio Cmobile We know elution time is related to amount of time in mobile phase- can we quantify this? Retention Time tr: IM Time- Fig 26-4 tR retention time for retained species average migration rate L column length tR tM time for unretained species(dead time) same rate as mobile phase molecules ayerage rate migration uL dead time tM Ideally.tR independent of volume injected produces Gaussian peaks CEM 333 page 14.5
(A) Adjusting Migration Rates: Analyte A in equilibrium with two phases Amobile « Astationary K = cstationary cmobile partition ratio We know elution time is related to amount of time in mobile phase - can we quantify this? Retention Time tR: Fig 26-4 tR retention time for retained species average migration rate v = L tR column length tM time for unretained species (dead time) same rate as mobile phase molecules average rate migration u = L tM dead time Ideally • tR independent of volume injected • produces Gaussian peaks CEM 333 page 14.5

Retention Time (tR)and Partition Ratio (K) Migration rate of analyte is VA u x fraction time A spends in mobile phase ux#mols A in mobile phase total mols A CMVM =u× CMVM+CSVS 1 =ux1+CsVs/CMVM =uX1+KA(Vs/VM) estimate from column packing Capacity Factor k'A: kA=KA(Vs/VM) [unitless for analyte A v=ux-1 1+kA How is k'A related to tr and tm? L-Lx 1 tR tM 1+kA k4=&-w tM When k'A is 30,separation is slow When k'A is 2-10,separation is optimum CEM 333 page 14.6
Retention Time (tR) and Partition Ratio (K): Migration rate of analyte is v A = u ´ fraction time A spends in mobile phase = u ´ # mols A in mobile phase total # mols A = u ´ cMVM cMVM + cSVS = u ´ 1 1+ cSVS / cMVM = u ´ 1 1+ KA (VS / VM ) estimate from column packing Capacity Factor k'A: kA ' = KA (VS / VM ) [unitless] for analyte A v = u ´ 1 1+ kA ' How is k'A related to tR and tM? L tR = L tM ´ 1 1+ kA ' kA ' = tR - tM tM When k'A is £1.0, separation is poor When k'A is >30, separation is slow When k'A is 2-10, separation is optimum CEM 333 page 14.6

Relative Migration Rates-Selectivity Factor (a): How do we compare elution of two components A and B? selectivity factor a= KB partition ratios KA KA capacity factors tR(B)-tM retention times tR(A)-tM larger a better separation (B)Adjusting Zone Broadening: Individual molecule undergoes"random walk" Many thousands of adsorption/desorption processes Average time for each step with some +ve or -ve differences Add up to give Gaussian peak (like random errors) Breadth of band increases down column because more time Zone broadening is affected by separation efficiency-more efficient,less broadening CEM 333 page 14.7
Relative Migration Rates - Selectivity Factor (a): How do we compare elution of two components A and B? selectivity factor a = KB KA partition ratios = kB ' kA ' capacity factors = tR(B) - tM tR(A) - tM retention times larger a = better separation (B) Adjusting Zone Broadening: • Individual molecule undergoes "random walk" • Many thousands of adsorption/desorption processes • Average time for each step with some +ve or -ve differences • Add up to give Gaussian peak (like random errors) • Breadth of band increases down column because more time • Zone broadening is affected by separation efficiency - more efficient, less broadening CEM 333 page 14.7

Column Efficiency: number of plates length of column N height of 1 theoretical plate Plates are only theoretical-column efficiency increases with N Analyte profile at end of packing (b) (L-1o) (L+1o) L Distance migrated (a) Packing Sample in Detector Fig 26-5 CEM333 page 14.8
Column Efficiency: number of plates length of column N = L H height of 1 theoretical plate Plates are only theoretical - column efficiency increases with N Fig 26-5 CEM 333 page 14.8
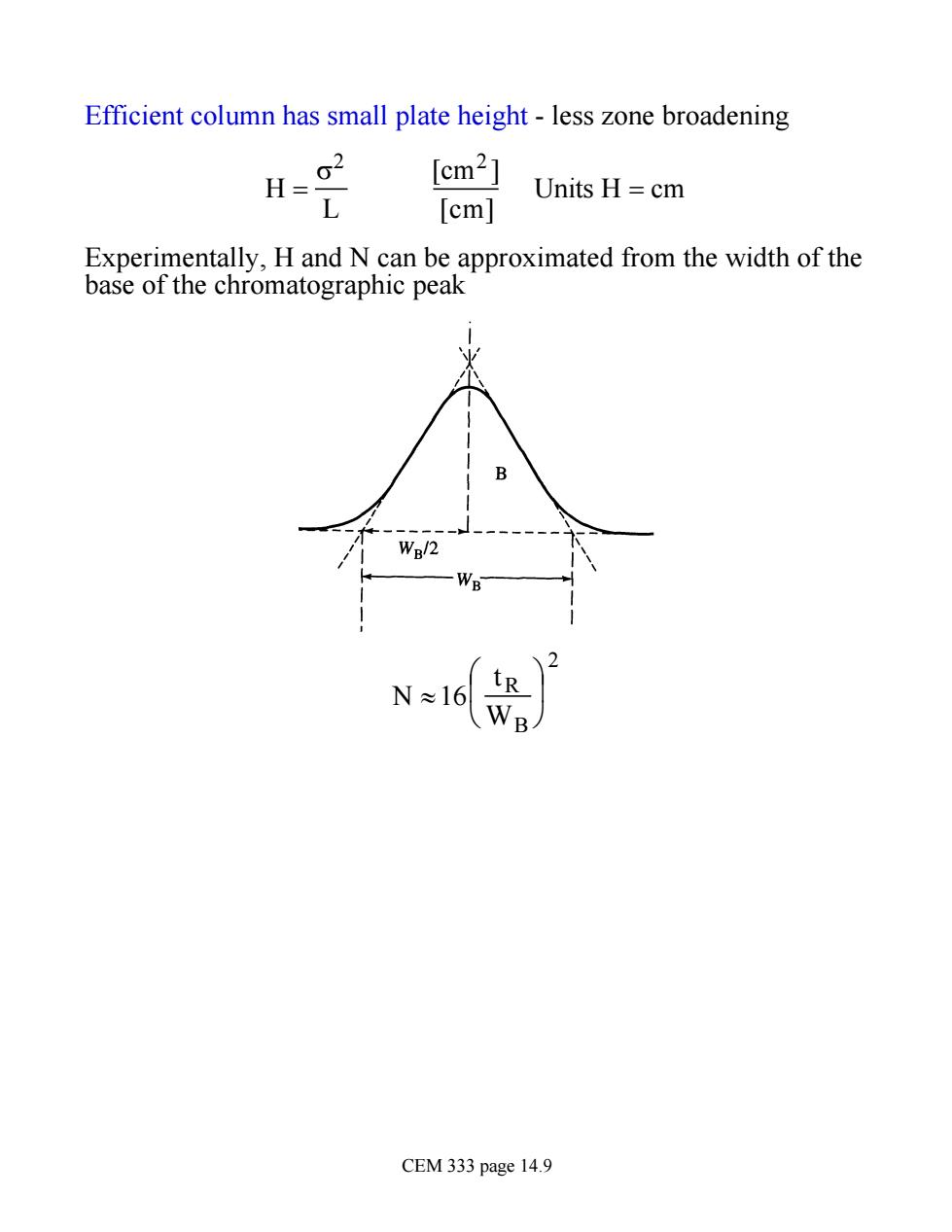
Efficient column has small plate height-less zone broadening H=g2 [cm2] L Units H=cm [cm] Experimentally,H and N can be approximated from the width of the base of the chromatographic peak 2 L D N≈1 6 CEM 333 page 14.9
Efficient column has small plate height - less zone broadening H = s 2 L [cm2 ] [cm] Units H = cm Experimentally, H and N can be approximated from the width of the base of the chromatographic peak N »16 tR WB æ è ç ö ø ÷ 2 CEM 333 page 14.9

Other Variables Affecting Peak Width(Zone Broadening): Mobile Phase Velocity: Higher mobile phase velocity,less time on column.less zone broadening However,plate height H also changes with flow rate-plot of H versus u called van Deemter plot (Fig 26-7) 8.0 7.0 4.0 3.0 0 2.0 4.0 6.0 8.0 Linear flow rate,cm/s CEM 333 page 14.10
Other Variables Affecting Peak Width (Zone Broadening): Mobile Phase Velocity: Higher mobile phase velocity, less time on column, less zone broadening However, plate height H also changes with flow rate - plot of H versus u called van Deemter plot (Fig 26-7) CEM 333 page 14.10