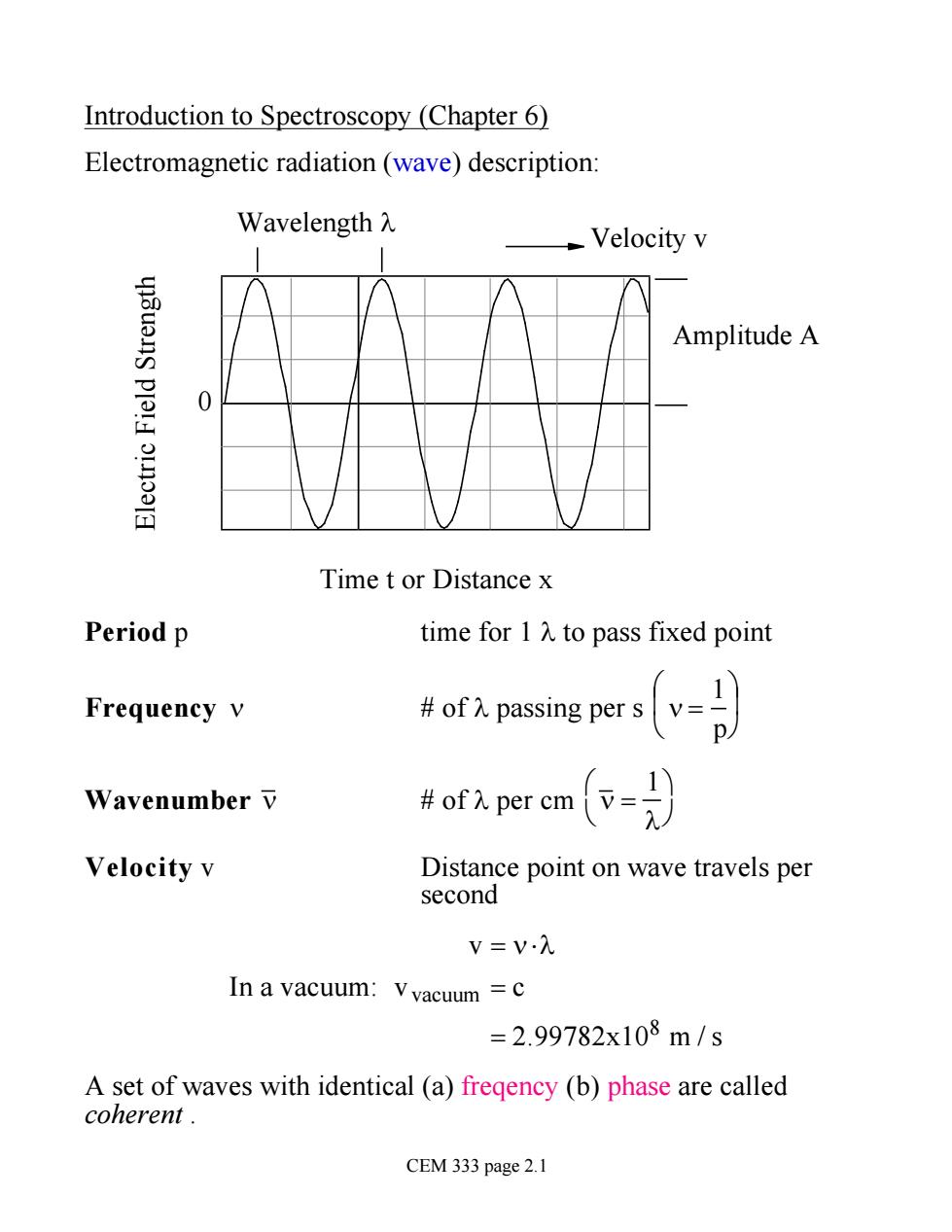
Introduction to Spectroscopy (Chapter 6) Electromagnetic radiation(wave)description: Wavelength入 Velocity v Amplitude A Time t or Distance x Period p time for 1 A to pass fixed point 1 Frequency v of passing per sv= p 1 Wavenumber v #of入per cm Velocity v Distance point on wave travels per second V=V入 In a vacuum:Vvacuum=c =2.99782x108m/s A set of waves with identical (a)fregency (b)phase are called coherent. CEM 333 page 2.1
Introduction to Spectroscopy (Chapter 6) Electromagnetic radiation (wave) description: Electric Field Strength Time t or Distance x Amplitude A Velocity v Wavelength l 0 Period p time for 1 l to pass fixed point Frequency n # of l passing per s n = 1 p æ è ç ö ø ÷ Wavenumber n # of l per cm n = 1 l æ è ö ø Velocity v Distance point on wave travels per second In a vacuum: v = n ×l vvacuum = c = 2.99782x108 m / s A set of waves with identical (a) freqency (b) phase are called coherent . CEM 333 page 2.1

Frequency is always fixed but velocity can vary! Waves slow down in medium(gas,liquid,solid)so v<c V=Vλ Implies A decreases in medium Refractive index1.00 Equation for Wave E=Asin(ot+Φ)where o=2πv electric field frequency amplitude phase angular frequency time Wave description explains certain EM radiation phenomena: transmission reflection and refraction diffraction interference scattering polarization CEM 333 page 2.2
Frequency is always fixed but velocity can vary! Waves slow down in medium (gas, liquid, solid) so v<c v = n ×l Implies l decreases in medium Refractive index h = c v ³ 1.00 Equation for Wave E = Asin(wt + f) where w = 2pn electric field frequency amplitude phase angular frequency time Wave description explains certain EM radiation phenomena: transmission reflection and refraction diffraction interference scattering polarization CEM 333 page 2.2
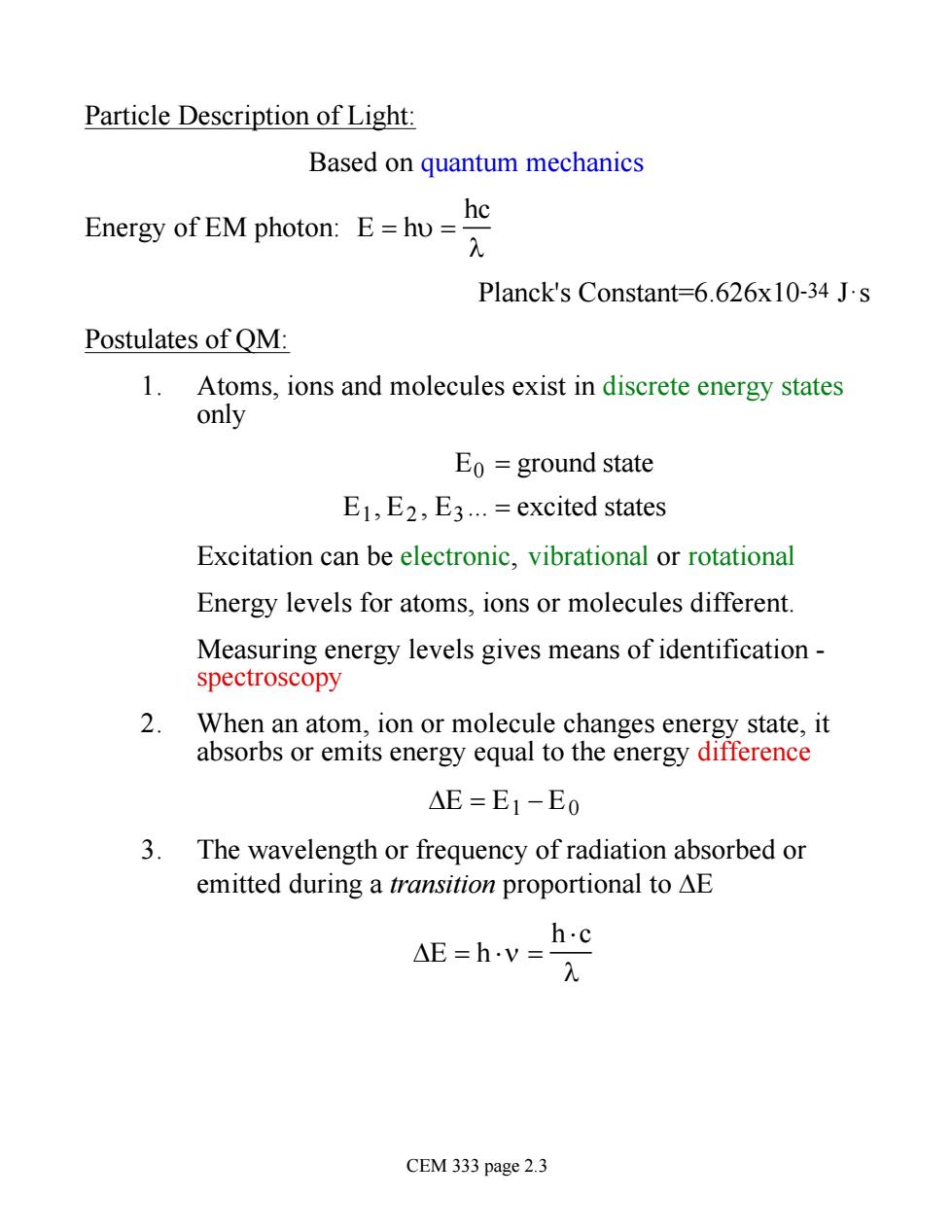
Particle Description of Light: Based on quantum mechanics Energy of EM photon:E=huhe Planck's Constant=6.626x10-34 J.s Postulates of QM 1.Atoms,ions and molecules exist in discrete energy states only Eo=ground state E1,E2,E3.excited states Excitation can be electronic,vibrational or rotational Energy levels for atoms,ions or molecules different. Measuring energy levels gives means of identification- spectroscopy When an atom,ion or molecule changes energy state,it absorbs or emits energy equal to the energy difference AE=E1-Eo 3.The wavelength or frequency of radiation absorbed or emitted during a transition proportional to AE h.c AE=h.v= CEM 333 page 2.3
Particle Description of Light: Based on quantum mechanics Energy of EM photon: E = hu = hc l Planck's Constant=6.626x10-34 J·s Postulates of QM: 1. Atoms, ions and molecules exist in discrete energy states only E0 = ground state E1 , E2 , E3 . = excited states Excitation can be electronic, vibrational or rotational Energy levels for atoms, ions or molecules different. Measuring energy levels gives means of identification - spectroscopy 2. When an atom, ion or molecule changes energy state, it absorbs or emits energy equal to the energy difference DE = E1 - E0 3. The wavelength or frequency of radiation absorbed or emitted during a transition proportional to DE DE = h ×n = h ×c l CEM 333 page 2.3
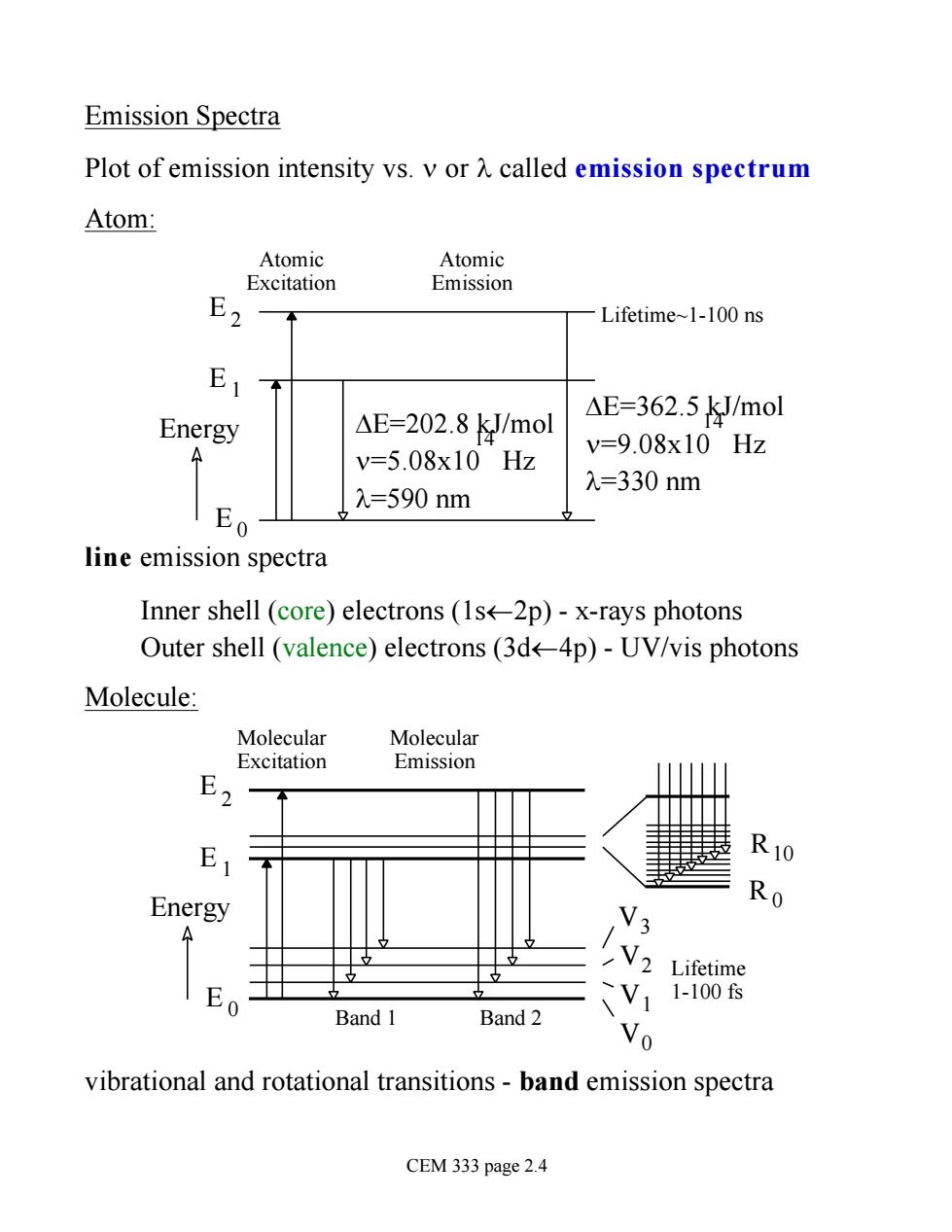
Emission Spectra Plot of emission intensity vs.v or A called emission spectrum Atom: Atomic Atomic Excitation Emission Lifetime~1-100 ns △E=362.5kJ/mol Energy △E=202.8kJ/mol v=9.08x10Hz v=5.08x10Hz \E0 =590nm 入=330nm line emission spectra Inner shell (core)electrons (1s<-2p)-x-rays photons Outer shell (valence)electrons(3d-4p)-UV/vis photons Molecule: Molecular Molecular Excitation 七m1ss1on R10 Energy Ro V3 V2 Lifetime V11-1006 Band 1 Band 2 vibrational and rotational transitions-band emission spectra CEM 333 page 2.4
Emission Spectra Plot of emission intensity vs. n or l called emission spectrum Atom: E 0 E 1 E 2 DE=202.8 kJ/mol n=5.08x10 Hz l=590 nm 14 DE=362.5 kJ/mol n=9.08x10 Hz l=330 nm 14 Energy Atomic Excitation Atomic Emission Lifetime~1-100 ns line emission spectra Inner shell (core) electrons (1s¬2p) - x-rays photons Outer shell (valence) electrons (3d¬4p) - UV/vis photons Molecule: E 0 E 1 E 2 Energy Molecular Excitation Molecular Emission V0 V1 V2 V3 R0 R10 Band 1 Band 2 Lifetime 1-100 fs vibrational and rotational transitions - band emission spectra CEM 333 page 2.4
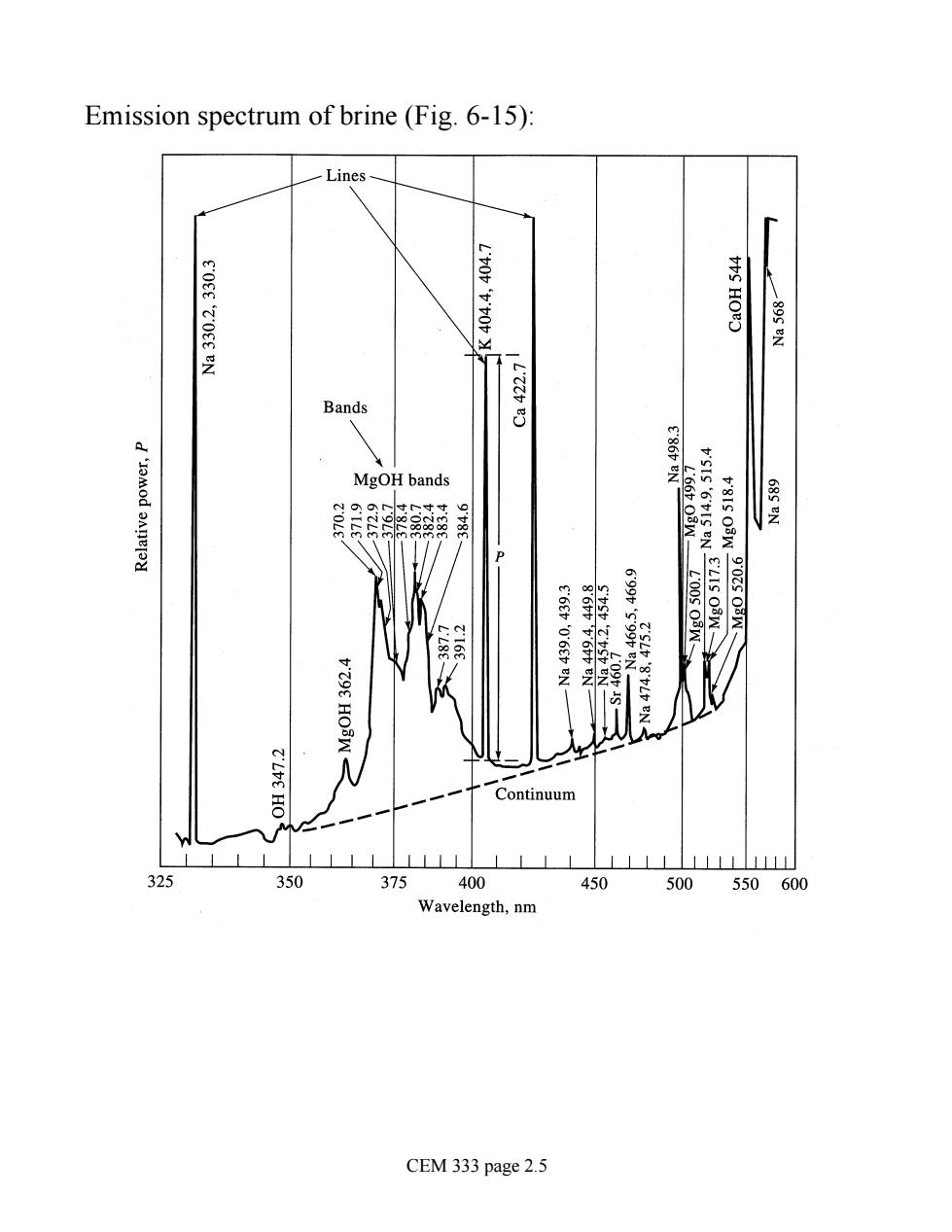
Emission spectrum of brine(Fig.6-15): Bands 22588 MgOH bands 0050- E'LIS O3W- 02s0aN- Continuum 325 350 325 400 450 500 550600 Wavelength,nm CEM 333 page 2.5
Emission spectrum of brine (Fig. 6-15): CEM 333 page 2.5
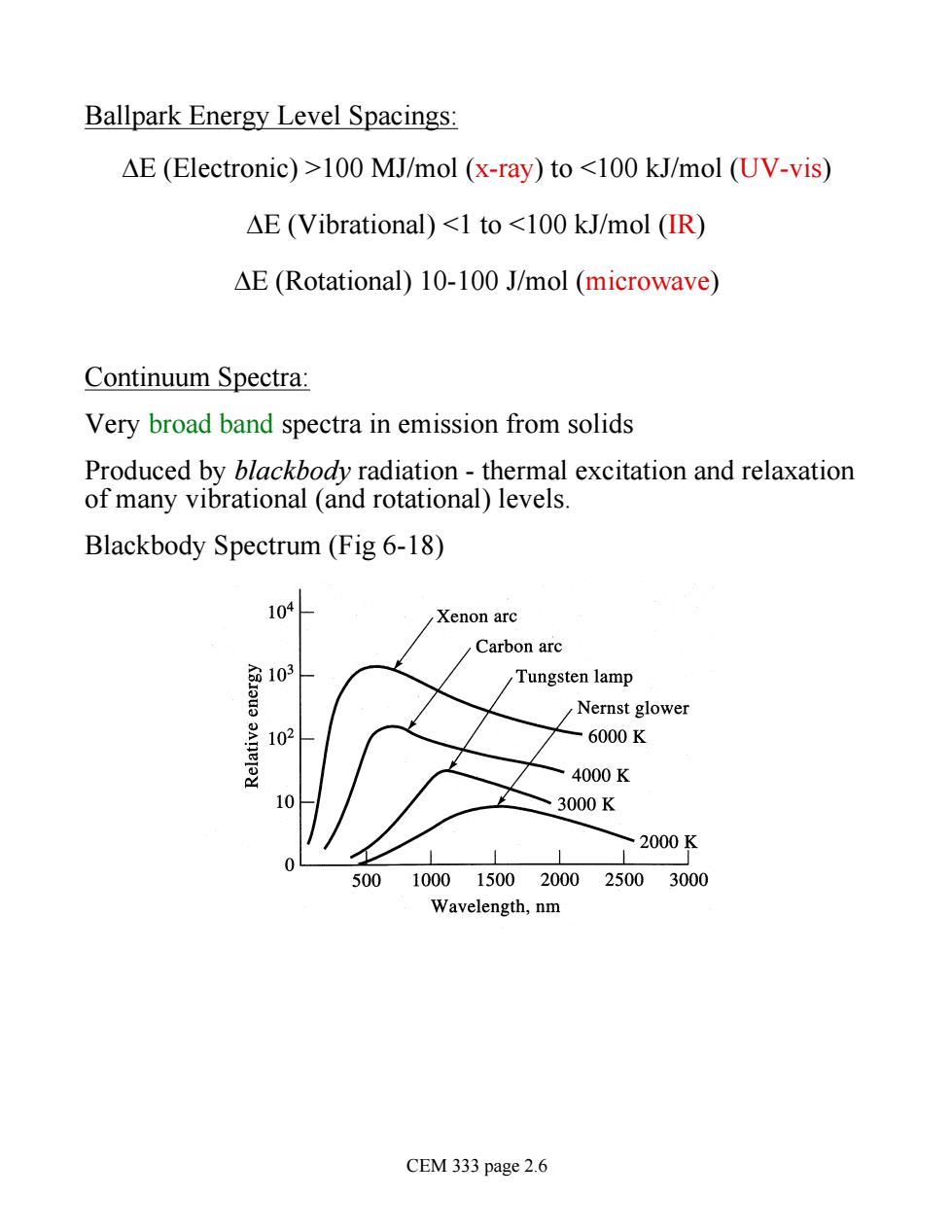
Ballpark Energy Level Spacings AE (Electronic)>100 MJ/mol (x-ray)to <100 kJ/mol (UV-vis) AE (Vibrational)<1 to <100 kJ/mol (IR) AE (Rotational)10-100 J/mol (microwave) Continuum Spectra: Very broad band spectra in emission from solids Produced by blackbody radiation -thermal excitation and relaxation of many vibrational (and rotational)levels. Blackbody Spectrum (Fig 6-18) 104 Xenon arc Carbon arc 103 Tungsten lamp Nernst glower -6000K 4000K 3000K / 2000K 50010001500200025003000 Wavelength,nm CEM 333 page 2.6
Ballpark Energy Level Spacings: DE (Electronic) >100 MJ/mol (x-ray) to <100 kJ/mol (UV-vis) DE (Vibrational) <1 to <100 kJ/mol (IR) DE (Rotational) 10-100 J/mol (microwave) Continuum Spectra: Very broad band spectra in emission from solids Produced by blackbody radiation - thermal excitation and relaxation of many vibrational (and rotational) levels. Blackbody Spectrum (Fig 6-18) CEM 333 page 2.6

Absorption Spectra Plot of Absorbance vs.v or called absorption spectrum Just as in emisson spectra an atom,ion or molecule can only absorb radiation if energy matches separation between two energy states Atoms: No vibrational or rotational energy levels-sharp line spectra with few features For example Na 3s->3p 589.0,589.6 nm (yellow) Na3s-→5p285.0,285.1nm(UV) Visible enough energy for valence (bonding)excitations UV and x-ray enough energy for core(inner)excitations Molecules: Electronic,vibrational and rotational energy levels-broad band spectra with many features AE=AEelectronie+AEvibrational +AErotational For each electronic state-many vibrational states For each vibrational state -many rotational states -→many features Absorption spectra affected by (1)number of atoms in molecule (2)solvent molecules more features blurred features CEM 333 page 2.7
Absorption Spectra Plot of Absorbance vs. n or l called absorption spectrum Just as in emisson spectra an atom, ion or molecule can only absorb radiation if energy matches separation between two energy states Atoms: No vibrational or rotational energy levels - sharp line spectra with few features For example: Na 3s®3p 589.0, 589.6 nm (yellow) Na 3s®5p 285.0, 285.1 nm (UV) Visible enough energy for valence (bonding) excitations UV and x-ray enough energy for core (inner) excitations Molecules: Electronic, vibrational and rotational energy levels - broad band spectra with many features DE = DEelectronic + DEvibrational + DErotational For each electronic state - many vibrational states For each vibrational state - many rotational states ®many features Absorption spectra affected by (1) number of atoms in molecule (2) solvent molecules more features blurred features CEM 333 page 2.7

Effect of Chemical State(Fig 6-19): (a)Na vapor 0 588 589 590 (b)Benzene vapo (c)Benzene in hexane (d)Biphenyl in hexane 220 260 300 340 Wavelength,nm CEM 333 page 2.8
Effect of Chemical State (Fig 6-19): CEM 333 page 2.8
Relaxation Processes: Lifetime of excited state is short (fs->ms)-relaxational processes return excited species to ground state Nonradiative relaxation many small collisional relaxations tiny temperature rise of surrounding species Radiative relaxation (emission) fluorescence (10-5 s) Resonance fluorescence produces emission at same energy/frequency/wavelength as absorption common for atoms(no V or R levels) Non-resonance fluorescence produces emission at lower energy (lower frequency/longer wavelength)than absorption (Stokes shift) common in molecules -vibrational relaxation occurs before fluorescence Phosphorescence Produced by long-lived electronic state (up to hours) CEM 333 page 2.9
Relaxation Processes: Lifetime of excited state is short (fs®ms) - relaxational processes return excited species to ground state Nonradiative relaxation many small collisional relaxations tiny temperature rise of surrounding species Radiative relaxation (emission) fluorescence (10-5 s) Resonance fluorescence produces emission at same energy/frequency/wavelength as absorption common for atoms (no V or R levels) Non-resonance fluorescence produces emission at lower energy (lower frequency/longer wavelength) than absorption (Stokes shift) common in molecules - vibrational relaxation occurs before fluorescence Phosphorescence Produced by long-lived electronic state (up to hours) CEM 333 page 2.9

Absorption Nonradiative Fluorescence relaxation E2 UV Vis R Eo Excitation methods: (① EM radiation (ii) spark/discharge/arc Gii) particle bombardment (electrons,ions. (iv) chemiluminescence (exothermic chemical reaction generates excited products) CEM 333 page 2.10
IR Vis UV Absorption Nonradiative relaxation Fluorescence Resonance Non-resonance E 2 E 1 E 0 Excitation methods: (i) EM radiation (ii) spark/discharge/arc (iii) particle bombardment (electrons, ions. ) (iv) chemiluminescence (exothermic chemical reaction generates excited products) CEM 333 page 2.10