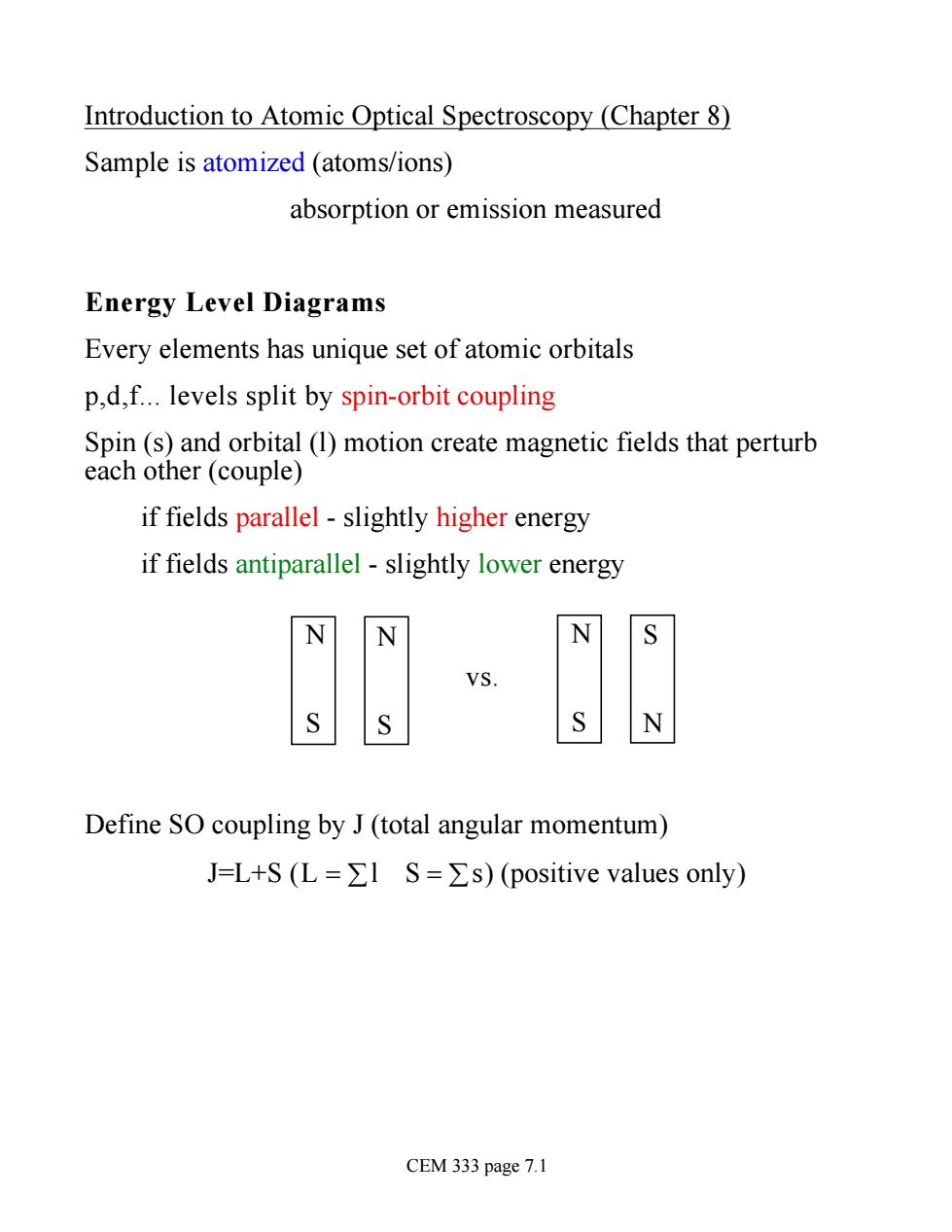
Introduction to Atomic Optical Spectroscopy (Chapter 8) Sample is atomized (atoms/ions) absorption or emission measured Energy Level Diagrams Every elements has unique set of atomic orbitals p,d,f.levels split by spin-orbit coupling Spin(s)and orbital (1)motion create magnetic fields that perturb each other (couple) if fields parallel-slightly higher energy if fields antiparallel-slightly lower energy N N VS. N Define SO coupling by J(total angular momentum) J=L+S(L=ΣlS=Σs)(positive values only) CEM 333 page 7.1
Introduction to Atomic Optical Spectroscopy (Chapter 8) Sample is atomized (atoms/ions) absorption or emission measured Energy Level Diagrams Every elements has unique set of atomic orbitals p,d,f. levels split by spin-orbit coupling Spin (s) and orbital (l) motion create magnetic fields that perturb each other (couple) if fields parallel - slightly higher energy if fields antiparallel - slightly lower energy N S N S vs. N S S N Define SO coupling by J (total angular momentum) J=L+S (L = ål S = ås) (positive values only) CEM 333 page 7.1
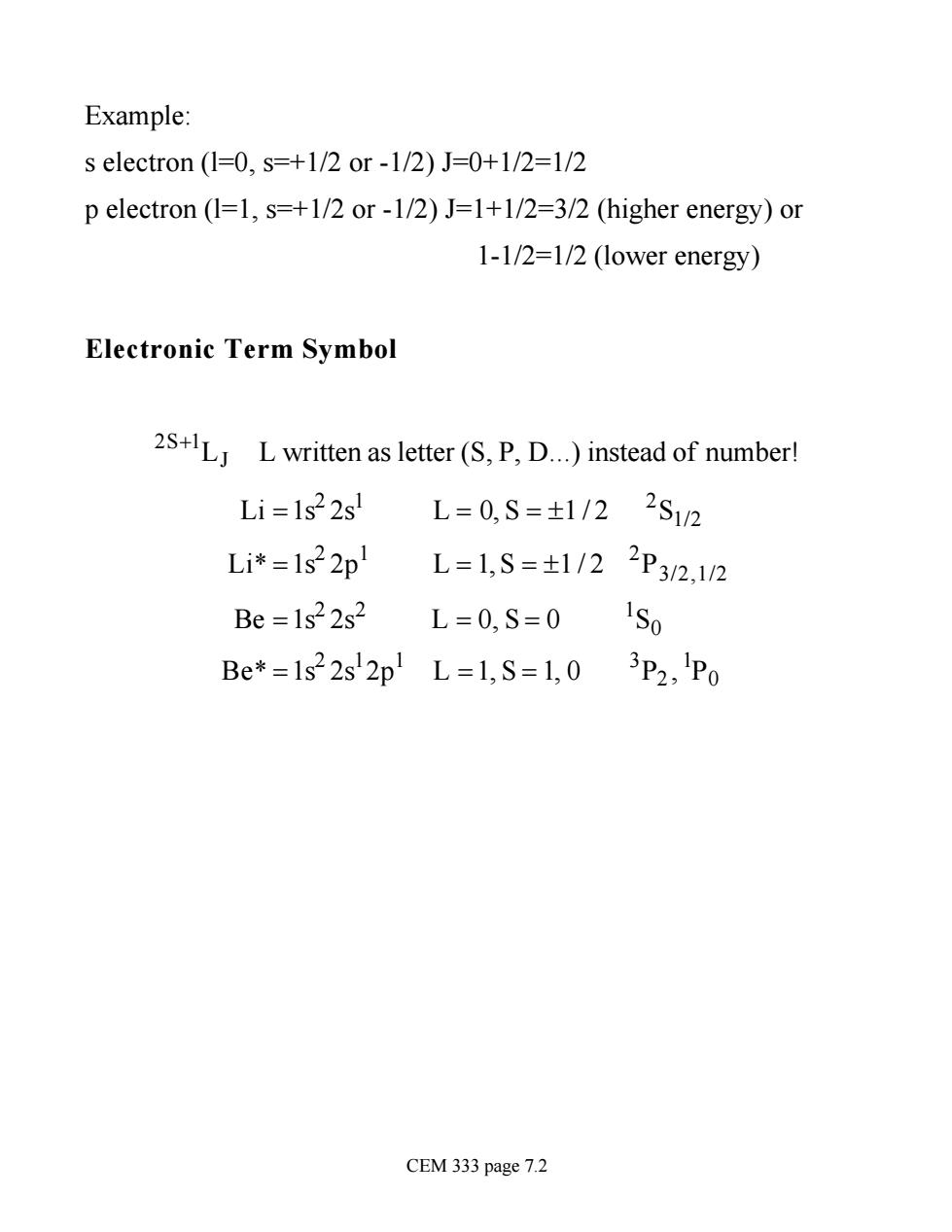
Example: s electron(1=0,s=+1/2or-1/2)J=0+1/2=l/2 p electron (1=1,s=+1/2 or-1/2)J=1+1/2=3/2(higher energy)or 1-1/2=1/2 (lower energy) Electronic Term Symbol 2+LLwritten as letter(S,P,D.)instead of number! Li=1s22s! L=0,S=±1/22S12 Li*=1s22p L=1,S=t1/22P32,12 Be =1s22s2 L=0,S=0 1S Be*=1s22s2p1L=1,S=1,03P2,1P0 CEM 333 page 7.2
Example: s electron (l=0, s=+1/2 or -1/2) J=0+1/2=1/2 p electron (l=1, s=+1/2 or -1/2) J=1+1/2=3/2 (higher energy) or 1-1/2=1/2 (lower energy) Electronic Term Symbol 2S+1LJ L written as letter (S, P, D.) instead of number! Li =1s2 2s1 L = 0, S = ±1 / 2 2 S1/2 Li* =1s2 2p1 L = 1, S = ±1 / 2 2 P3/2,1/2 Be =1s2 2s2 L = 0, S = 0 1 S0 Be* =1s2 2s1 2p1 L =1, S = 1, 0 3 P2 , 1 P0 CEM 333 page 7.2
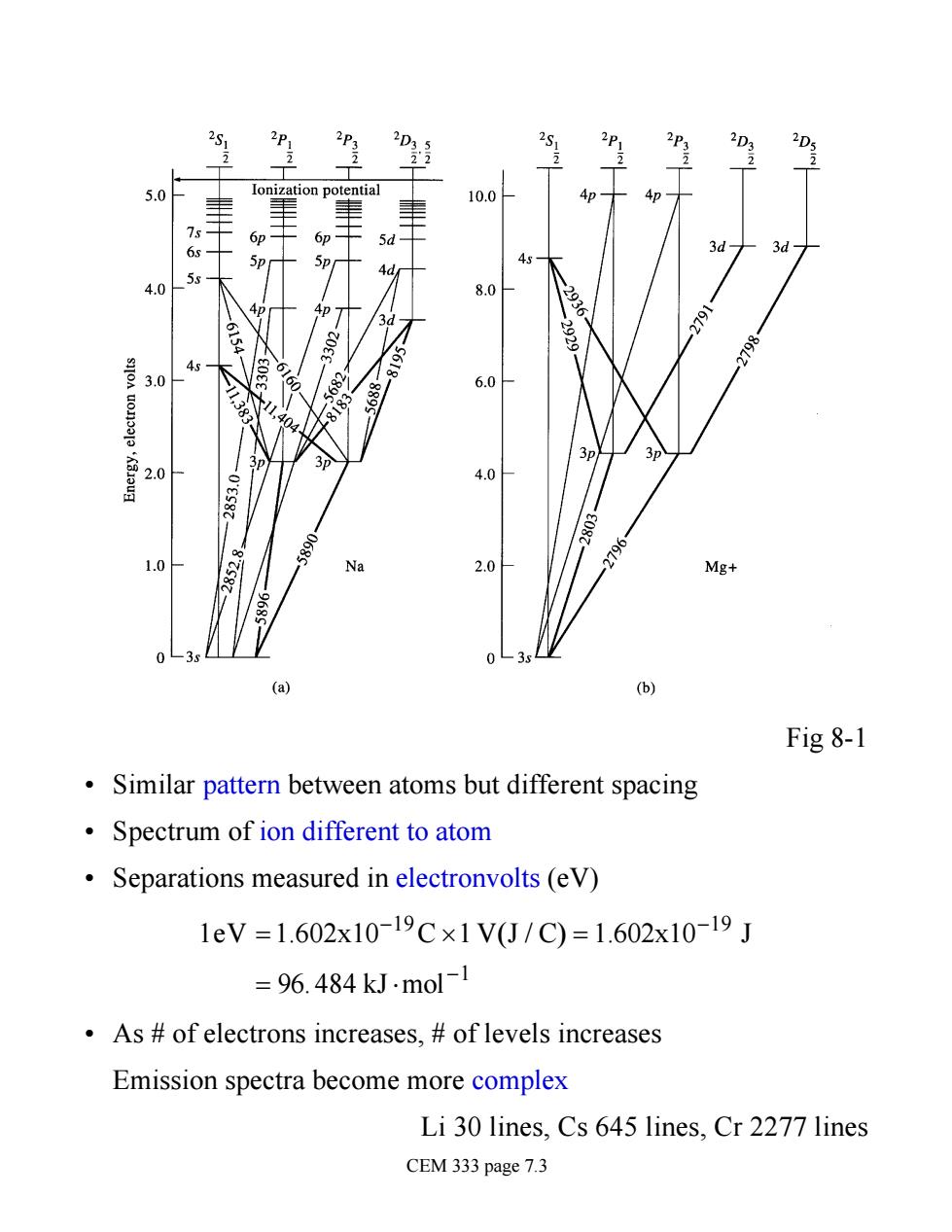
3P: D lonization potential 10.0 5d d 43 4.0 8.0 4p- 号30 45 682 81 20 2.0 Mg+ 0-3 (a) (b) Fig 8-1 Similar pattern between atoms but different spacing Spectrum of ion different to atom Separations measured in electronvolts (eV) 1eV=1.602x10-19c×1VJ/C)=1.602x10-19J =96.484 kJ.mol-1 As of electrons increases,of levels increases Emission spectra become more complex Li 30 lines,Cs 645 lines,Cr 2277 lines CEM 333 page 7.3
Fig 8-1 • Similar pattern between atoms but different spacing • Spectrum of ion different to atom • Separations measured in electronvolts (eV) 1eV =1.602x10-19C´1 V(J / C) = 1.602x10-19 J = 96. 484 kJ×mol-1 • As # of electrons increases, # of levels increases Emission spectra become more complex Li 30 lines, Cs 645 lines, Cr 2277 lines CEM 333 page 7.3

Desire narrow lines for accurate identification Broadened by (i)uncertainty principle (ii)pressure broadening (iii)Doppler effect (iv)(electric and magnetic fields) Atomic line widths: (i)Uncertainty Principle: Quantum mechanical idea states must measure for some minimum time to tell two frequencies apart △t·△E≥h △t △y≥1 minin measurement difference in frequencies Shows up in lifetime of excited state if lifetime infinitely long,AE infinitely narrow ·if lifetime short,△E is broadened CEM 333 page 7.4
Desire narrow lines for accurate identification Broadened by (i) uncertainty principle (ii) pressure broadening (iii) Doppler effect (iv) (electric and magnetic fields) Atomic line widths: (i) Uncertainty Principle: Quantum mechanical idea states must measure for some minimum time to tell two frequencies apart Dt×DE ³ h Dt minimum time for measurement { × Dn minimum detectable difference in frequencies { ³1 Shows up in lifetime of excited state • if lifetime infinitely long, DE infinitely narrow • if lifetime short, DE is broadened CEM 333 page 7.4
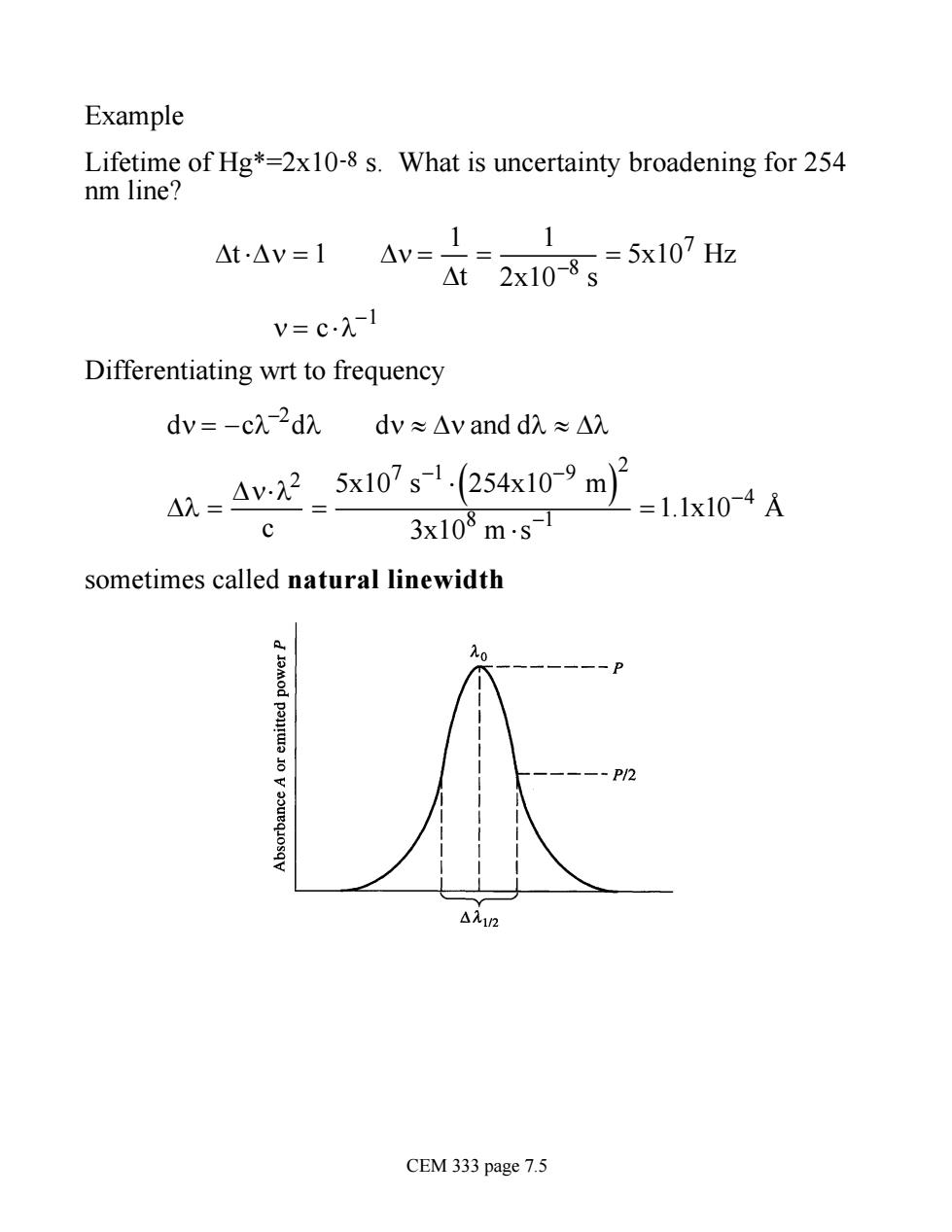
Example Lifetime of Hg*=2x10-8 s.What is uncertainty broadening for 254 nm line? At-Av=1 Av=1= At2x10-8g=5x107z 1 V=cλ-1 Differentiating wrt to frequency dv=-cλ-2ddv≈△y and dA≈△ .=4v22_5x107s1(254x10-9m2 3x108m.s-1 =1.1x104A c sometimes called natural linewidth △入12 CEM 333 page 7.5
Example Lifetime of Hg*=2x10-8 s. What is uncertainty broadening for 254 nm line? Dt ×Dn = 1 Dn = 1 Dt = 1 2x10-8 s = 5x107 Hz n = c ×l-1 Differentiating wrt to frequency dn = -cl -2 dl dn » Dn and dl » Dl Dl = Dn×l2 c = 5x107 s -1 × 254x10-9 ( m) 2 3x108 m ×s -1 =1.1x10-4 Å sometimes called natural linewidth CEM 333 page 7.5

(ii)Pressure broadening: Collisions with atoms/molecules transfers small quantities of vibrational energy (heat)-ill-defined ground state energy Effect worse at high pressures For low pressure hollow cathode lamps(1-10 torr)10-1-10-2 A For high pressure Xe lamps(>10,000 torr)100-1000 A(turns lines into continua!) (iii)Doppler broadening Change in frequency produced by motion relative to detector In gas,broadens line symmetrically Doppler broadening increases with T ·At room T~10-2-10-3A Total linewidth typically 0.01-0.1 A CEM 333 page 7.6
(ii) Pressure broadening: Collisions with atoms/molecules transfers small quantities of vibrational energy (heat) - ill-defined ground state energy Effect worse at high pressures • For low pressure hollow cathode lamps (1-10 torr) 10-1-10-2 Å • For high pressure Xe lamps (>10,000 torr) 100-1000 Å (turns lines into continua!) (iii) Doppler broadening: Change in frequency produced by motion relative to detector In gas, broadens line symmetrically Doppler broadening increases with T • At room T ~10-2-10-3 Å Total linewidth typically 0.01-0.1 Å CEM 333 page 7.6
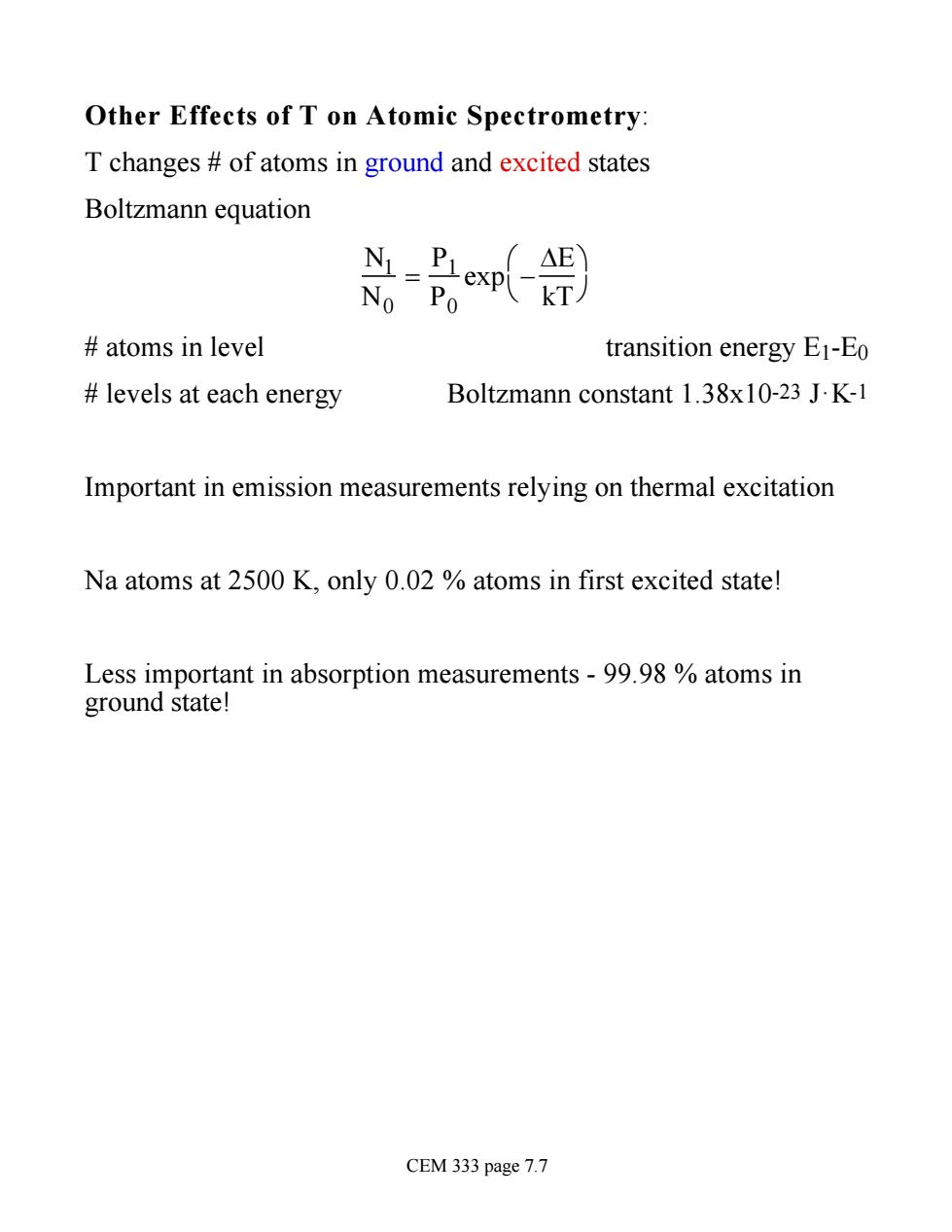
Other Effects of T on Atomic Spectrometry T changes of atoms in ground and excited states Boltzmann equation T△E No Po exp(kT) atoms in level transition energy E1-Eo levels at each energy Boltzmann constant 1.38x10-23 J.K-1 Important in emission measurements relying on thermal excitation Na atoms at 2500 K,only 0.02 atoms in first excited state! Less important in absorption measurements -99.98 atoms in ground state! CEM 333 page 7.7
Other Effects of T on Atomic Spectrometry: T changes # of atoms in ground and excited states Boltzmann equation N1 N0 = P1 P0 exp - DE kT æ è ö ø # atoms in level transition energy E1-E0 # levels at each energy Boltzmann constant 1.38x10-23 J·K-1 Important in emission measurements relying on thermal excitation Na atoms at 2500 K, only 0.02 % atoms in first excited state! Less important in absorption measurements - 99.98 % atoms in ground state! CEM 333 page 7.7
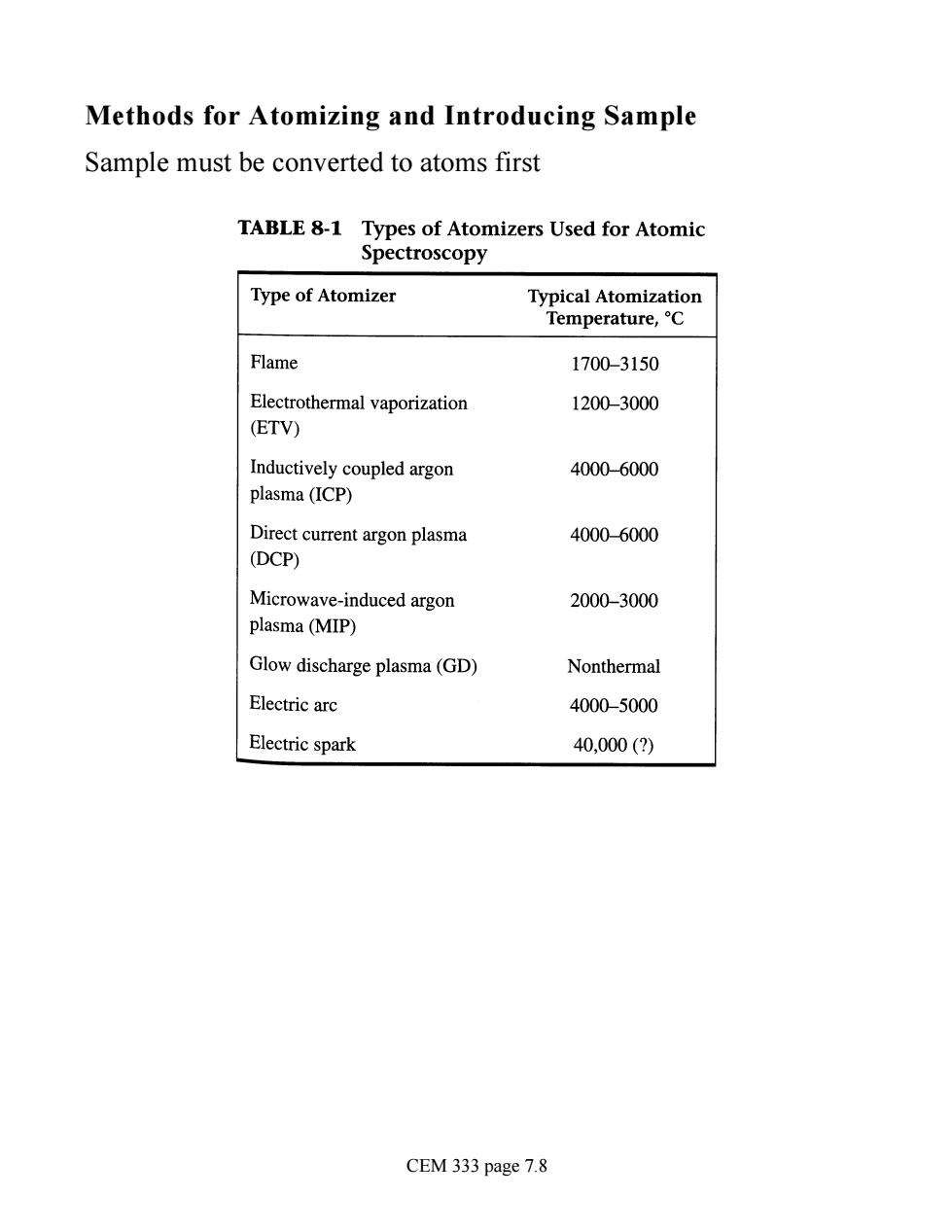
Methods for Atomizing and Introducing Sample Sample must be converted to atoms first TABLE 8-1 Types of Atomizers Used for Atomic Spectroscopy Type of Atomizer Typical Atomization Temperature,C Flame 1700-3150 Electrothermal vaporization 1200-3000 (ETV) Inductively coupled argon 4000-6000 plasma(ICP) Direct current argon plasma 4000-6000 (DCP) Microwave-induced argon 2000-3000 plasma(MIP) Glow discharge plasma(GD) Nonthermal Electric arc 4000-5000 Electric spark 40,000(2) CEM 333 page 7.8
Methods for Atomizing and Introducing Sample Sample must be converted to atoms first CEM 333 page 7.8

Must transfer sample to atomizer-easy for gases/solutions but difficult for solids TABLE 8-2 Methods of Sample Introduction in Atomic Spectroscopy Method Type of Sample Pneumatic nebulization Solution or slurry Ultrasonic nebulization Solution Electrothermal vaporization Solid,liquid,solution Hydride generation Solution of certain elements Direct insertion Solid,powder Laser ablation Solid,metal Spark or arc ablation Conducting solid Glow discharge sputtering Conducting solid CEM 333 page 7.9
Must transfer sample to atomizer - easy for gases/solutions but difficult for solids CEM 333 page 7.9