
PHYSICAL STATE OF INGREDIENTS IN FOOD SYSTEMS Food Dispersions 1. True solution 2. Colloidal dispersion 3. Emulsion 4. Foam 5. Gel Dispersions 1. Continuous phase 2. Dispersed phase May be solid, liquid, or gas
PHYSICAL STATE OF INGREDIENTS IN FOOD SYSTEMS Food Dispersions 1. True solution 2. Colloidal dispersion 3. Emulsion 4. Foam 5. Gel Dispersions 1. Continuous phase 2. Dispersed phase May be solid, liquid, or gas

True Solution The dispersion of particle < 1 nm in liquid. Examples: sugar, lactose, minerals, and vitamins. Colloidal Dispersion (SOL) Dispersion of particle sizes between 10-100 nm in liquid. Common colloids: dispersion of proteins, large molecular salts. Example: milk
True Solution The dispersion of particle < 1 nm in liquid. Examples: sugar, lactose, minerals, and vitamins. Colloidal Dispersion (SOL) Dispersion of particle sizes between 10-100 nm in liquid. Common colloids: dispersion of proteins, large molecular salts. Example: milk

Emulsions Liquid/liquid systems of 2 immiscible substances are called emulsion. Substances or particle size = 10-100 microns. Examples: butter (w/o), margarine (w/o), mayonnaise (o/w), salad dressing (o/w), milk (o/w), cream (o/w), and chip-dip (o/w). Water Oil Oil Oil Oil Oil H O H O H O H O Oil/Water Water/Oil 2 2 2 2 Oil Oil Oil Oil Oil Oil
Emulsions Liquid/liquid systems of 2 immiscible substances are called emulsion. Substances or particle size = 10-100 microns. Examples: butter (w/o), margarine (w/o), mayonnaise (o/w), salad dressing (o/w), milk (o/w), cream (o/w), and chip-dip (o/w). Water Oil Oil Oil Oil Oil H O H O H O H O Oil/Water Water/Oil 2 2 2 2 Oil Oil Oil Oil Oil Oil
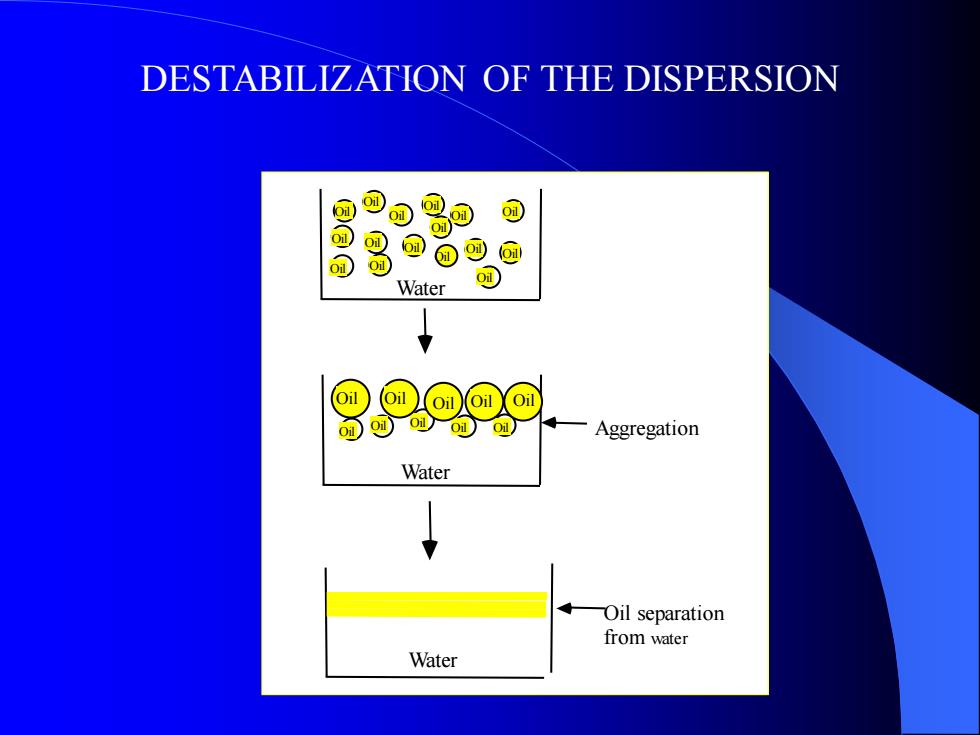
DESTABILIZATION OF THE DISPERSION Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Water Water Oil Oil Oil Oil Oil Water Oil Aggregation Oil separation from water
DESTABILIZATION OF THE DISPERSION Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Oil Water Water Oil Oil Oil Oil Oil Water Oil Aggregation Oil separation from water
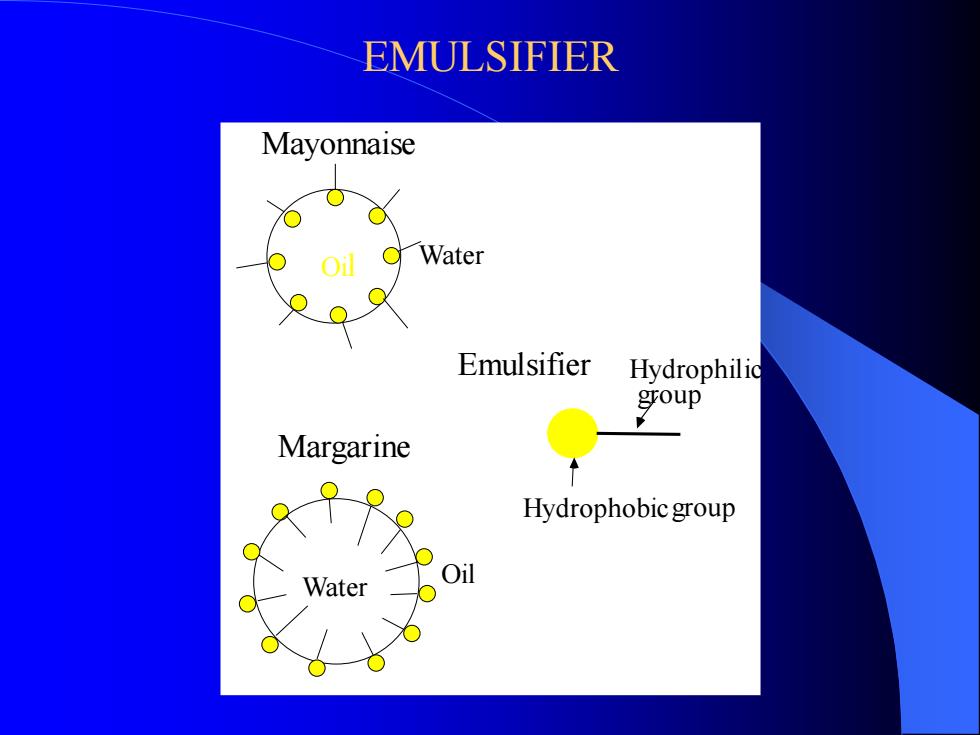
EMULSIFIER Oil Water Mayonnaise Water Oil Margarine Emulsifier Hydrophobicgroup Hydrophilic group
EMULSIFIER Oil Water Mayonnaise Water Oil Margarine Emulsifier Hydrophobicgroup Hydrophilic group
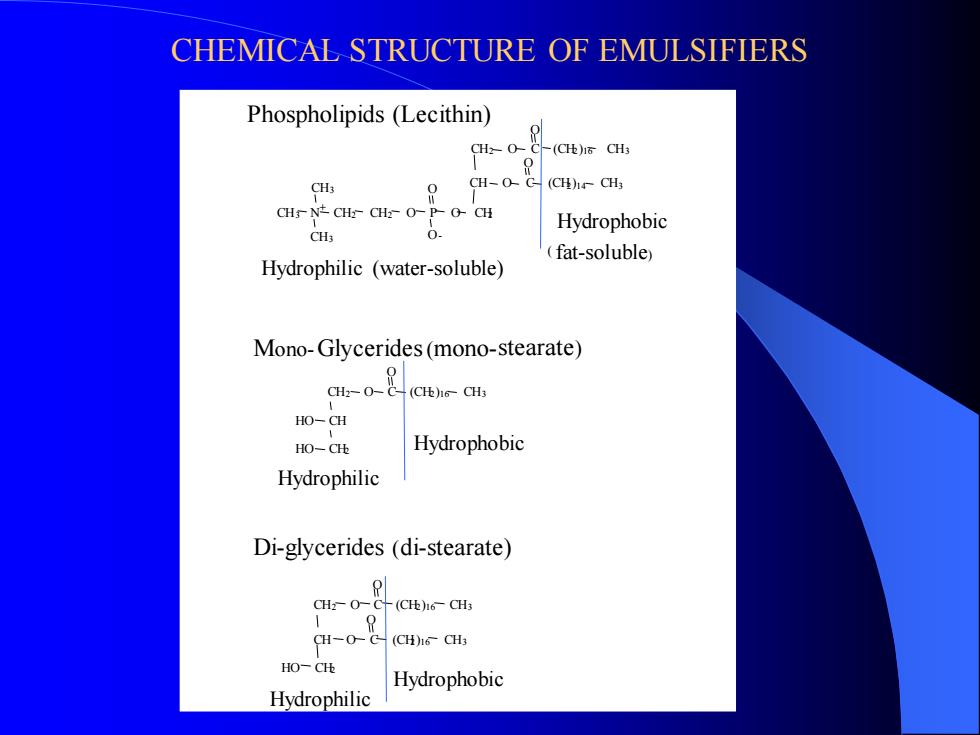
CHEMICAL STRUCTURE OF EMULSIFIERS CH3 CH3 CH3 CH2 O C (CH2)16 CH3 O O N CH2 CH2 O P O CH2 O CH O C (CH2)14 CH3 O CH2 O C (CH2)16 CH3 HO CH HO CH2 O CH2 O C (CH2)16 CH3 CH O C (CH2)16 CH3 HO CH2 O O Phospholipids (Lecithin) Hydrophobic ( fat-soluble) Hydrophilic (water-soluble) Mono-Glycerides(mono-stearate) Hydrophobic Hydrophilic Di-glycerides (di-stearate) Hydrophobic Hydrophilic - +
CHEMICAL STRUCTURE OF EMULSIFIERS CH3 CH3 CH3 CH2 O C (CH2)16 CH3 O O N CH2 CH2 O P O CH2 O CH O C (CH2)14 CH3 O CH2 O C (CH2)16 CH3 HO CH HO CH2 O CH2 O C (CH2)16 CH3 CH O C (CH2)16 CH3 HO CH2 O O Phospholipids (Lecithin) Hydrophobic ( fat-soluble) Hydrophilic (water-soluble) Mono-Glycerides(mono-stearate) Hydrophobic Hydrophilic Di-glycerides (di-stearate) Hydrophobic Hydrophilic - +

O C CH CH 2 O C (CH 2)16 CH 3 C CH C H2 H O OH H OH HO O OH O H2 O C CH CH 2 O C (CH 2)16 CH 3 C CH C O CH 2 CH 2 O CH 2 CH 2OH CH2 CH2 O CH 2 CH 2OH Span 60 (sorbitan mono-stearate) Hydrophobic Hydrophilic Tween 60 (polyoxyalkylene sorbitan mono-stearate) Hydrophobic Hydrophilic
O C CH CH 2 O C (CH 2)16 CH 3 C CH C H2 H O OH H OH HO O OH O H2 O C CH CH 2 O C (CH 2)16 CH 3 C CH C O CH 2 CH 2 O CH 2 CH 2OH CH2 CH2 O CH 2 CH 2OH Span 60 (sorbitan mono-stearate) Hydrophobic Hydrophilic Tween 60 (polyoxyalkylene sorbitan mono-stearate) Hydrophobic Hydrophilic

SOME DESIRABLE CHARACTERISTICS OF FOOD EMULSIFIERS 1. Ability to reduce interfacial tension below 10 dynes/cm 2. Ability to be rapidly absorbed at the interface 3. Ability to function effectively at low concentrations 4. Resistance to chemical change 5. Lack of odor, color, and toxicity 6. Economical
SOME DESIRABLE CHARACTERISTICS OF FOOD EMULSIFIERS 1. Ability to reduce interfacial tension below 10 dynes/cm 2. Ability to be rapidly absorbed at the interface 3. Ability to function effectively at low concentrations 4. Resistance to chemical change 5. Lack of odor, color, and toxicity 6. Economical

FOAM Gas is dispersed in liquid or semi-liquid. Dispersed-phase: gas Continuous-phase: liquid It requires a 3rd component possessing protective or stabilizing properties to maintain the dispersion. Example: whipped topping
FOAM Gas is dispersed in liquid or semi-liquid. Dispersed-phase: gas Continuous-phase: liquid It requires a 3rd component possessing protective or stabilizing properties to maintain the dispersion. Example: whipped topping

The important foam stability factors are: 1. Surface tension 2. Concentration of separate phase 3. Presence of foaming agent to lower surface tension 4. Viscosity of liquid - the higher the viscosity, the more stable the foam. 5. Presence and thickness of adsorption layer (a 3rd stabilizing material)
The important foam stability factors are: 1. Surface tension 2. Concentration of separate phase 3. Presence of foaming agent to lower surface tension 4. Viscosity of liquid - the higher the viscosity, the more stable the foam. 5. Presence and thickness of adsorption layer (a 3rd stabilizing material)