
Protein Denaturation z Any modification in conformation not accompanied by rupture of peptide bonds z Ultimate step might correspond to a totally unfolded polypeptide structure z Reversible or irreversible
Protein Denaturation z Any modification in conformation not accompanied by rupture of peptide bonds z Ultimate step might correspond to a totally unfolded polypeptide structure z Reversible or irreversible
Effects of Denaturation z Decreased solubility z Altered water binding capacity z Loss of biological activity z Destruction of toxins z Improved digestibility z Increased intrinsic viscosity z Inability to crystallize
Effects of Denaturation z Decreased solubility z Altered water binding capacity z Loss of biological activity z Destruction of toxins z Improved digestibility z Increased intrinsic viscosity z Inability to crystallize
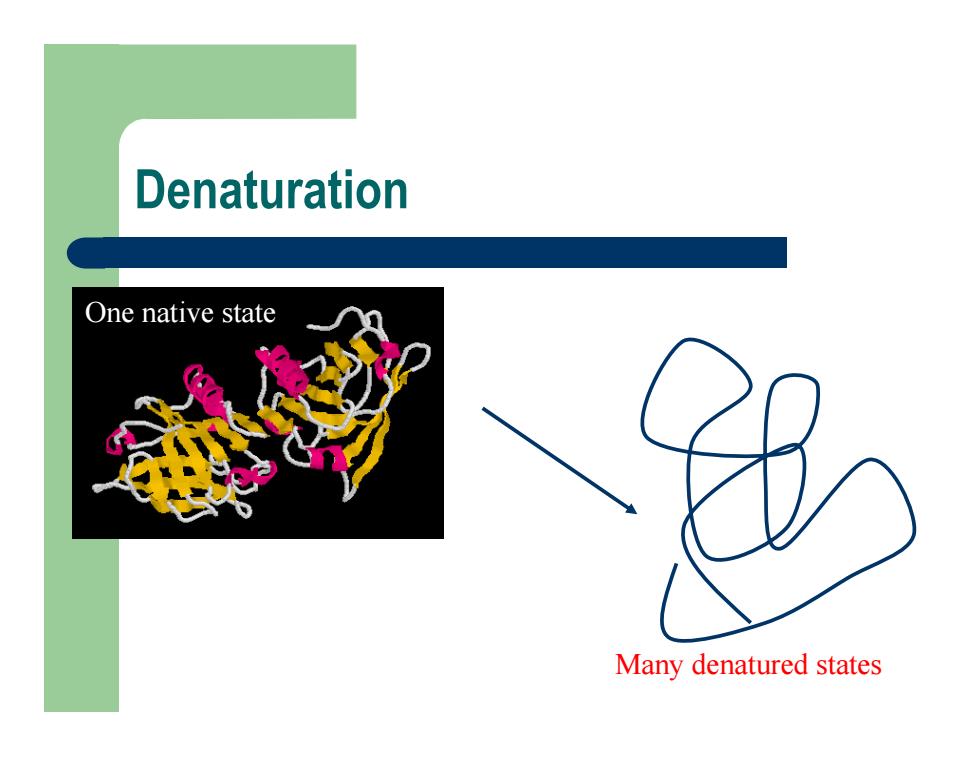
Denaturation One native state Many denatured states
Denaturation One native state Many denatured states
Physical Agents
Physical Agents
Thermal Denaturation z Rate of denaturation depends on the temperature z As T is increased – Affect interactions of tertiary structure – Increased flexibility → reversible – H-bonds begin to break → water interaction – Increased water binding – Increased viscosity of solution – Structures different from native protein
Thermal Denaturation z Rate of denaturation depends on the temperature z As T is increased – Affect interactions of tertiary structure – Increased flexibility → reversible – H-bonds begin to break → water interaction – Increased water binding – Increased viscosity of solution – Structures different from native protein
Thermal Denaturation z Upon cooling – Aggregation – Loss of solubility z Water content affects heat denaturation z Other consequences – Splitting of disulfide bonds – Chemical alterations of amino residues – Inter- or Intra- crosslinks
Thermal Denaturation z Upon cooling – Aggregation – Loss of solubility z Water content affects heat denaturation z Other consequences – Splitting of disulfide bonds – Chemical alterations of amino residues – Inter- or Intra- crosslinks
Effect of Cold Temperatures z Can result in denaturation – Gliadins, egg and milk proteins z Remain active – Some lipases and oxidases – Release from sub-cellular compartments z Proteins with high hydrophobic/polar amino residues and structures dependent on hydrophobic interactions
Effect of Cold Temperatures z Can result in denaturation – Gliadins, egg and milk proteins z Remain active – Some lipases and oxidases – Release from sub-cellular compartments z Proteins with high hydrophobic/polar amino residues and structures dependent on hydrophobic interactions
Interfaces z Liquid-air or Liquid-liquid interfaces z If allowed at interfaces – Unfold z Depends on – Rigidity of the 3-D structure – Number and location of hydrophobic groups – Accelerated if applied energy to cause shear z Reversible?
Interfaces z Liquid-air or Liquid-liquid interfaces z If allowed at interfaces – Unfold z Depends on – Rigidity of the 3-D structure – Number and location of hydrophobic groups – Accelerated if applied energy to cause shear z Reversible?

Others z Mechanical treatments z Hydrostatic Pressure z Irradiation
Others z Mechanical treatments z Hydrostatic Pressure z Irradiation
Chemical Agents
Chemical Agents