
Introduction to Food Science Chapter 6 Penfield and Campbell 1990
Introduction to Food Science Chapter 6 Penfield and Campbell 1990

Water The Universal Chemical
Water The Universal Chemical

Exits in Three States 1. Gas – Steam 2. Liquid – Water 3. Solid – Ice
Exits in Three States 1. Gas – Steam 2. Liquid – Water 3. Solid – Ice

Heat Transfer is necessary for conversion Conversion from: Water Vapor to Liquid Liquid to Solid Heat must be removed = Exothermic
Heat Transfer is necessary for conversion Conversion from: Water Vapor to Liquid Liquid to Solid Heat must be removed = Exothermic

Liquid to Vapor Solid to Liquid Heat must be supplied = Endothermic
Liquid to Vapor Solid to Liquid Heat must be supplied = Endothermic

Chemical Nature of Water Covalent bonds between O H H
Chemical Nature of Water Covalent bonds between O H H
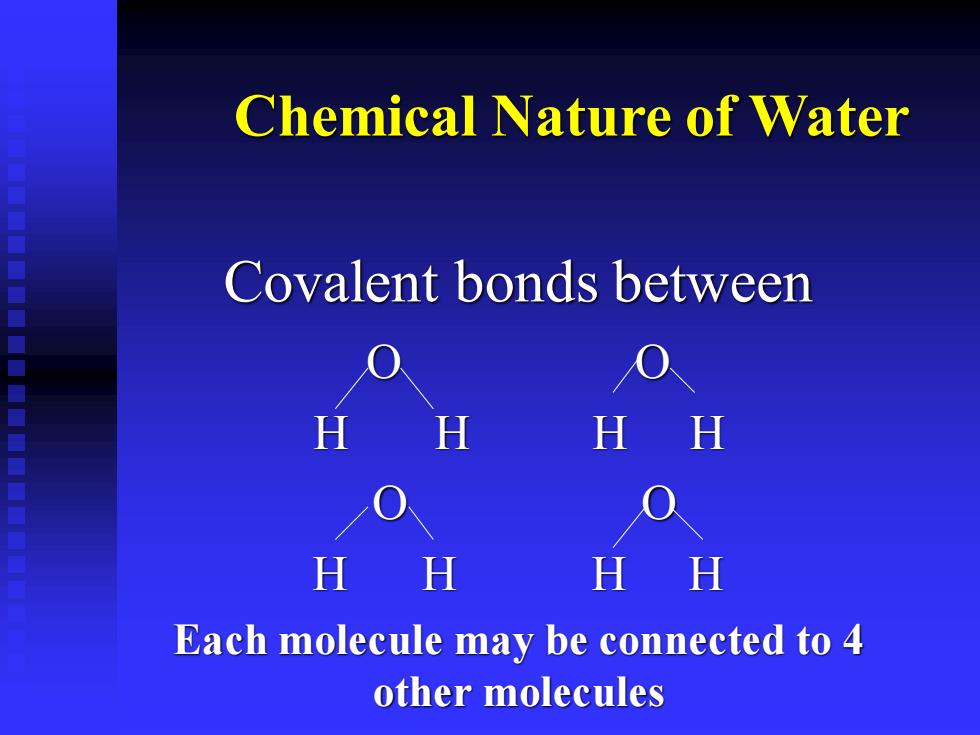
Chemical Nature of Water Covalent bonds between O O H H H H O O H H H H Each molecule may be connected to 4 other molecules
Chemical Nature of Water Covalent bonds between O O H H H H O O H H H H Each molecule may be connected to 4 other molecules

Natural Water is a solution
Natural Water is a solution

-Trapped in capillaries of food -Water holding capacity Water activity aw Water
-Trapped in capillaries of food -Water holding capacity Water activity aw Water

Water activity (aw) 1.00 – 0.95 Fresh Meat, fruit,vegetables 0.95 – 0.90 Processed cheese, baked goods 0.90 – 0.80 Aged cheese, and jams 0.80 - 0.70 Molasses, dried figs 0.70 - 0.60 Dried Fruit and corn syrup 0.60 - 0.50 Chocolate, honey, noodles 0.20 Dried milk, dried vegetable
Water activity (aw) 1.00 – 0.95 Fresh Meat, fruit,vegetables 0.95 – 0.90 Processed cheese, baked goods 0.90 – 0.80 Aged cheese, and jams 0.80 - 0.70 Molasses, dried figs 0.70 - 0.60 Dried Fruit and corn syrup 0.60 - 0.50 Chocolate, honey, noodles 0.20 Dried milk, dried vegetable