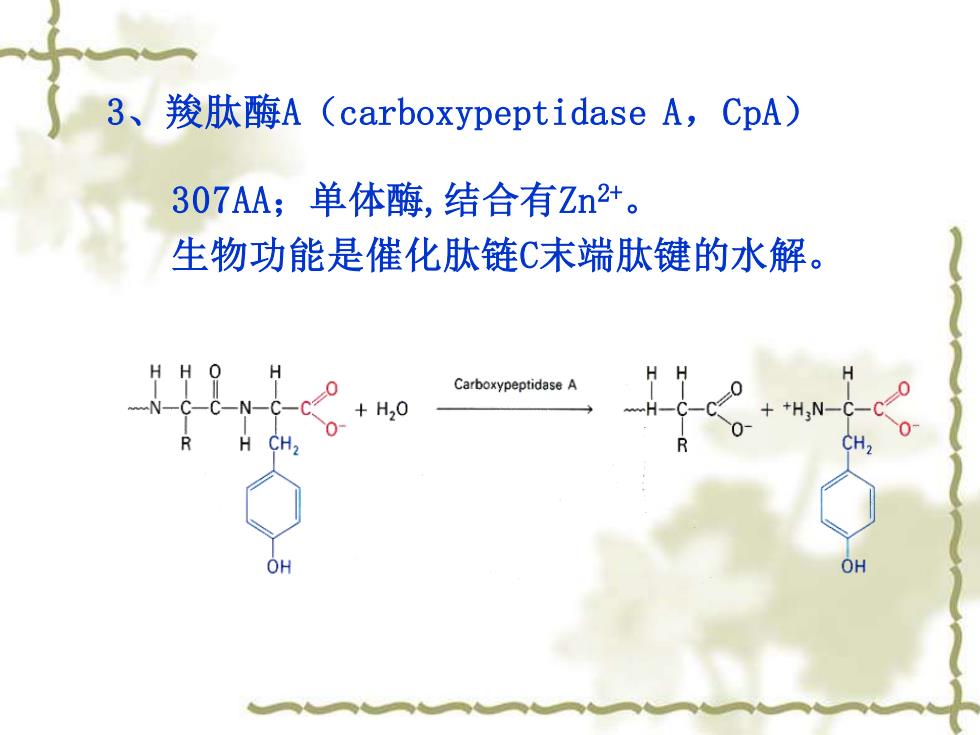
3、羧肽酶A(carboxypeptidase A,CpA) 307AA;单体酶,结合有Zn2+。 生物功能是催化肽链C末端肽键的水解。 Carboxypeptidase A +H20 ++H,N-C- CH2 OH OH
3、羧肽酶A(carboxypeptidase A,CpA) 307AA;单体酶,结合有Zn2+ 。 生物功能是催化肽链C末端肽键的水解

Zn2+ 60 307 140 120 Figure Three-dimensional structure of carboxypeptidase A.Only the a-carbon atoms and the zinc ion (shaded circle near the center)are shown.[From W.N.Lipscomb.Proc.Robert A. Welch Found.Conf.Chem.Res.15(1971):134.]
Zn2+
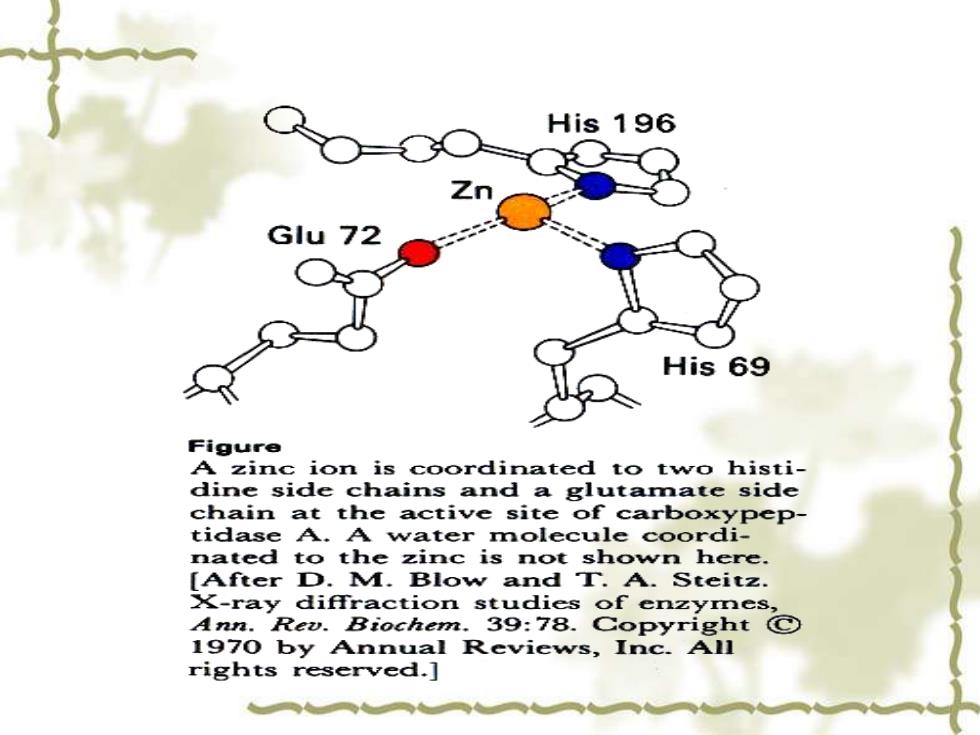
His 196 His 69 Figure A zinc ion is coordinated to two histi- dine side chains and a glutamate side chain at the active site of carboxypep- tidase A.A water molecule coordi- nated to the zinc is not shown here. [After D.M.Blow and T.A.Steitz. X-ray diffraction studies of enzymes, Ann.Rev.Biochem.39:78.Copyright C 1970 by Annual Reviews,Inc.All rights reserved.]

Nonpolar pocket +H2N NH Arg 145 Tyr 248 Glu 270 Figure Schematic representation of the bind- ing of glycyltyrosine to the active site of carboxypeptidase A.The pro- posed catalytically active complex is shown here
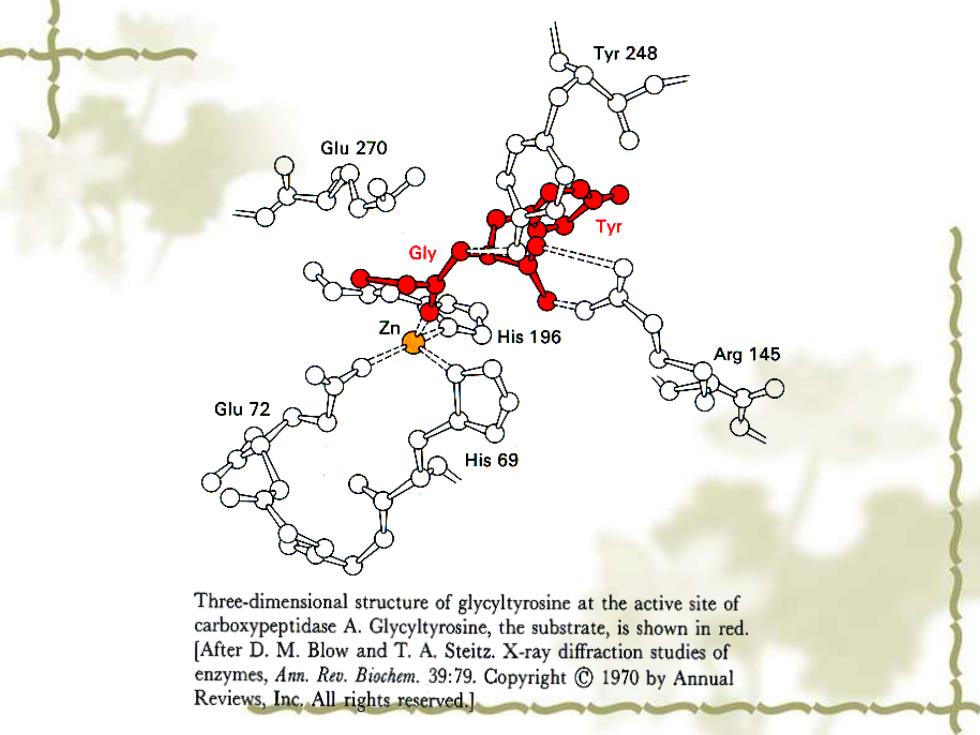
1 Tyr 248 Glu 270 ○His196 Arg 145 Glu 72 His 69 Three-dimensional structure of glycyltyrosine at the active site of carboxypeptidase A.Glycyltyrosine,the substrate,is shown in red. [After D.M.Blow and T.A.Steitz.X-ray diffraction studies of enzymes,Ann.Rev.Biochem.39:79.Copyright C 1970 by Annual Reviews,Inc.All rights reserved.]
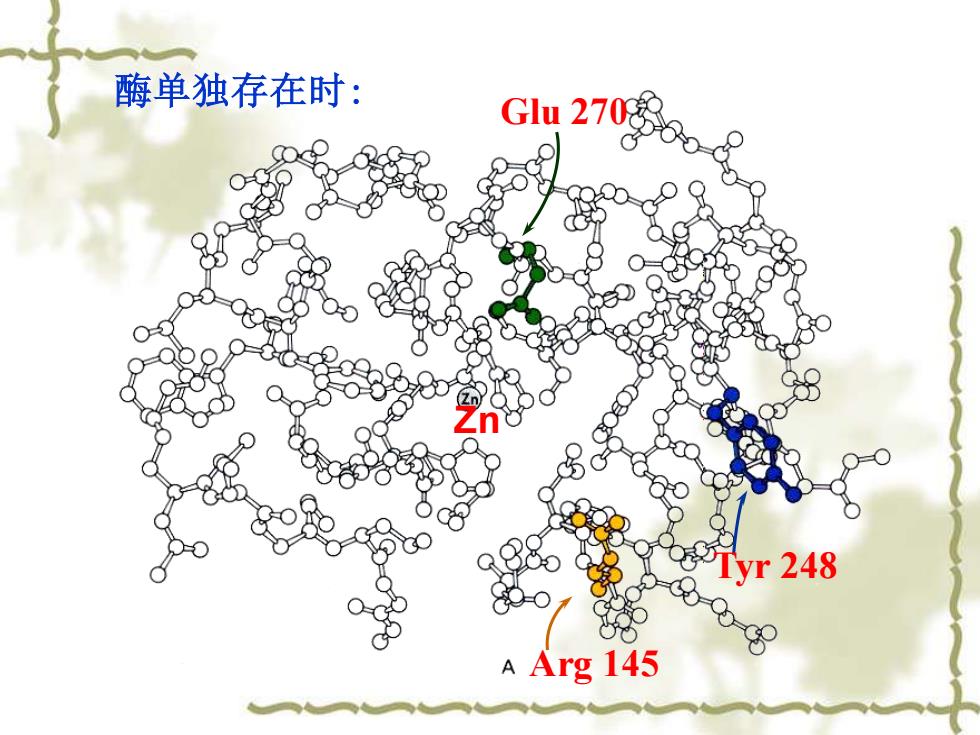
酶单独存在时: Glu 270 yr248 A Arg 145
Glu 270 Arg 145 Tyr 248 酶单独存在时: Zn
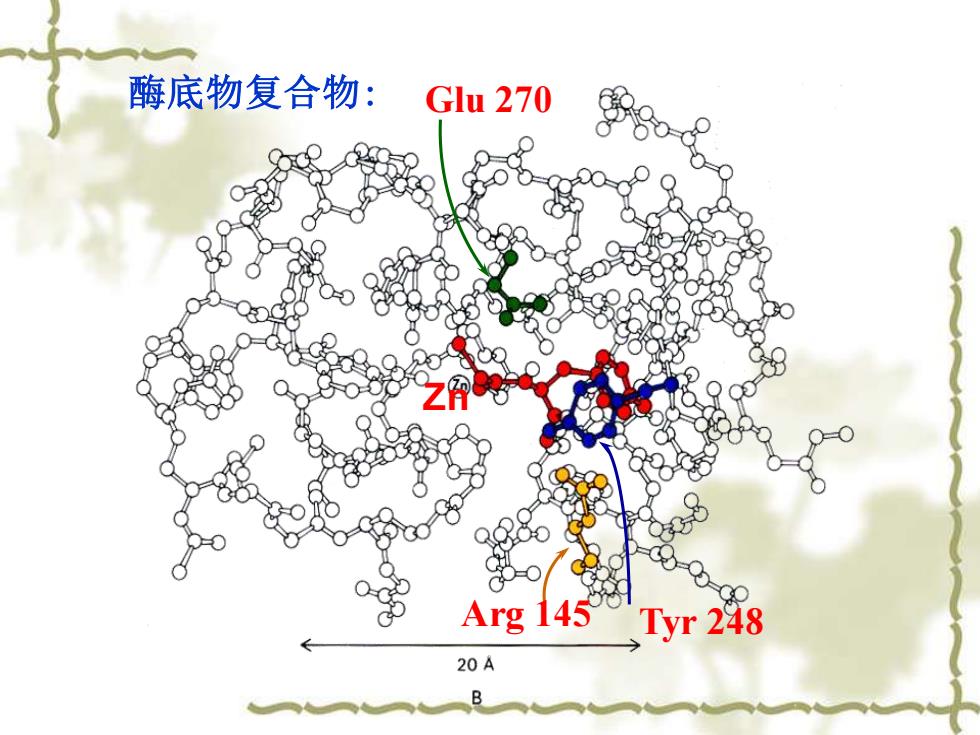
酶底物复合物: Glu 270 Arg 145Tyr 248 20A B
Arg 145 Tyr 248 酶底物复合物: Glu 270 Zn
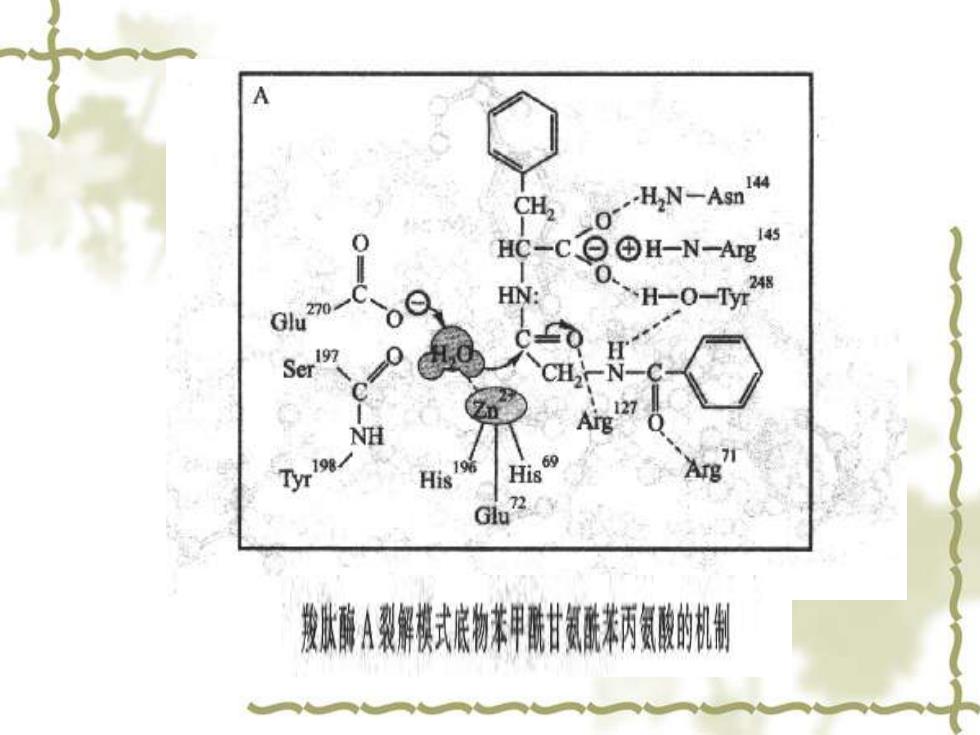
HC-C⊙©H-N-Ag6 Gly 270- oQ HN: 0 H-0-r28 CHN-C NH 96 71 His His Arg G2 段状炼A彩解摸式底物未甲甜氨酰柄氨酸的闲

B CH2 HN-Asn144 Gly27 145 HC-C百③H-N-A 248 NH .H-0-Tyr NH His His Arg 2 Glu' 发处游A裂察摸式接物未甲酰甘氨酰末丙资酸的机

CH -H2N一Asm4 HC-c H-N-Ag5 H N H-0-Tyr248 Gu270 H Ser 19 127 NH 72 Glu 图10-1我肤露A裂解摸式房物未甲张甘氨藏未柄贸酸的机制