
STUDY OF DISEASE AND PHYSIOLOGY IN THE 1979 HOMING STUDY HATCHERY STOCKS--A SUPPLEMENT TO:"IMPRINTING SALMON AND STEELHEAD TROUT FOR HOMING,1979 BY SLATICK,GILBREATH,AND WALCH" by Anthony J.Novotny and Waldo s.Zaugg Research Bonneville Power Administration (Contract DE-A179-79BP10682) and NoAA National Marine Fisheries Service a时 ine studies September 1981
STUDY OF DISEASE AND PHYSIOLOGY IN THE 1979 HOMING STUDY HATCHERY STOCKS--A SUPPLEMENT TO: "IMPRINTING SALMON AND STEELHEAD TROUT FOR HOMING, 1979 BY SLATICK, GILBREATH, AND WALCH" by Anthony J. Novotny and Waldo S. Zaugg Annual Report of Research Financed by Bonneville Power Administration (Contract DE-A179-79BP10682) and NOAA National Marine Fisheries Service Northwest and Alaska Fisheries Center Coastal Zone and Estuarine Studies Division 2725 Montlake Boulevard East Seattle, Washington 98112 September 1981

CONTENTS Page INTR0D0CTI0N… METHODS AND MATERIALS ................................................. 3 Hatchery Sampling 3 Sampling for Physiology........................................... plasma Electrolytes ......................................... 4 Gi11Wa-K'ATPase… Disease Sampling.................................................. Life History of Hatchery Juveniles .......................... 5 Blood Sample collection...................................... 5 Viral assays................................................. Histopathology… Bacteriological Assays....... 】 RESULTS AND DISCUSSION OF HATCHERY STEELHEAD SURVEYS................... 9 Chelan Hatchery (Transferred to Leavenworth Hatchery)Steelhead... Gill Nat-8t arease ................................................ plasma Electrolytes.......................................... 9 Hematology.................................................. Viral screening................................17 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease...............................17 Histopathology···························· 17 Seawater Adaptation
CONTENTS Page INTRODUCTION ........................................................... METHODS AND MATERIALS .................................................. Hatchery Sampling ................................................. Sampling for Physiology ........................................... Plasma Electrolytes .......................................... Gill Na+ -K+ ATPase ........................................... Disease Sampling .................................................. Life History of Hatchery Juveniles ........................... Blood Sample Collection ...................................... Viral Assays ................................................. Histopathology ............................................... Bacteriological Assays ....................................... RESULTS AND DISCUSSION OF HATCHERY STEELHEAD SURVEYS ................... Chelan Hatchery (Transferred to Leavenworth Hatchery) Steelhead ... Gill Na+-K+ ATPase ........................................... Plasma Electrolytes .......................................... Hematology ................................................... 1 3 3 4 4 4 5 5 5 7 7 7 9 9 9 9 13 Viral Screening . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17 Histopathology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17 Seawater Adaptation . . . . . . . . . . . . . . . . . . . . . . . . . . . 19

Wells Hatchery (Transferred to Winthrop Hatchery)Steelhead........19 Gill Na'-K'ATpase.................................................. 19 plasma electrolytes .......................................... 21 Hematology ................................................... 3 viral screening ................................................. 21 Indirect Fluorescent Antibody Test for Bacerial Kidney Digease.................................................................. 21 Histopathology................................................ 23 Seawater Adaptation............................................ 23 Tucannon Hatchery steelhead........................................ 23 Gi11a+-+ATPase… 23 p1 asma Electrolytes… 23 Hematol0gy… Viral screening................................................ 25 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease.................................................... Histopathology................................................... 25 Seawater Adaptation........................................... Sumary ........................................................... 29 RESULTS AND DISCUSSION OF HATCHERY CHINOOK SALMON SURVEYS· 34 Carson Hatchery Spring chinook Salmon.............................. 34 Gill Na'-K'ATPase… g plasma glectrolytes ............................................ Hematology........................................................... 哈 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease… 40
Wells Hatchery (Transferred to Winthrop Hatchery) Steelhead ........ 19 Gill Na+ -K+ ATPase ............................................ 19 Plasma Electrolytes ........................................... 21 Hematology .................................................... 21 Viral Screening ............................................... 21 Indirect Fluorescent Antibody Test for Bacerial Kidney Disease ....................................................... 21 Histopathology ................................................ 23 Seawater Adaptation ........................................... 23 Tucannon Hatchery Steelhead ........................................ 23 Gill Na+-K+ ATPase ............................................ 23 Plasma Electrolytes ........................................... 23 Hematology .................................................... 25 Viral Screening ............................................... 25 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease ....................................................... 25 Histopathology ................................................ 25 Seawater Adaptation ........................................... 28 Summary ............................................................ 29 RESULTS AND DISCUSSION OF HATCHERY CHINOOK SALMON SURVEYS ............... 34 Carson Hatchery Spring Chinook Salmon .............................. 34 Gill Na+ -K+ ATPase ............................................ 34 Plasma Electrolytes ........................................... 36 Hematology .................................................... 36 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease ....................................................... 40

Histopathology (See Appendix B)...............................40 Seawater Adaptation........................................... 42 Big White Salmon Hatchery Fall chinook salmon...................... 42 Gill Na'-K'ATpase............................................ Plasma Blectrolytes .......................................... 45 Hematology.................................................. Viral Screening… 45 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease....................................................... 45 Histopathology… 47 Seawater Adaptation… Summary ......................................................................... 49 ........................................................ 50 Stee1head.. % chinook salmon..................................................... 51 LITERATURE CITED 53 APPENDIX A APPENDIX B
Histopathology (See Appendix B) ............................... 40 Seawater Adaptation ........................................... 42 Big White Salmon Hatchery Fall Chinook Salmon ...................... 42 Gill Na+ -K+ ATPase ............................................ 42 Plasma Electrolytes ........................................... 45 Hematology .................................................... 45 Viral Screening ............................................... 45 Indirect Fluorescent Antibody Test for Bacterial Kidney Disease ....................................................... 45 Histopathology ................................................ 47 Seawater Adaptation ........................................... 47 Summary ............................................................ 49 CONCLUSIONS ............................................................. 50 Steelhead .......................................................... 50 Chinook Salmon ..................................................... 51 LITERATURE CITED ........................................................ 53 APPENDIX A APPENDIX B

INTRODUCTION The National Marine Fisheries Service (NMFS),under contract to the Bonneville Power Administration,is conducting research on imprinting salmon and steelhead for homing (Slatick et al.1979,1980;Novotny and Zaugg 1979).The studies were begun with little background knowledge of the effects of disease or certain physiological functions on imprinting and homing in salmonids.Consequently,work aimed at filling this void was begun by the authors in 1978 (Novotny and Zaugg 1979)and continued in 1979. In 1979,we examined random samples of normal populations of homing test fish at the hatcheries to determine the physiological readiness to migrate and adapt to seawater and general fish health.At the Manchester Marine Experimental Station,Manchester,Washington,we determined the survival of samples of the test fish maintained in marine net-pens after release from the hatcheries.Hatcheries and stocks sampled are listed in Table 1. The data collected from random samples were as follows: 1.Physiology. Gill Na+-K+ATPase.Abnormally low values could be indications that the fish were either in pre-or post-smolt condition,or had been stressed in some way. Plasma electrolytes.Lower than normal values of Na or cl could indicate immediate problems of osmoregulation when the fish were introduced to seawater;high values may indicate some dehydration due to stress. Increases in levels of K can indicate kidney failure or nitrogen supersaturation stresses
INTRODUCTION The National Marine Fisheries Service (NMFS), under contract to the Bonneville Power Administration, is conducting research on imprinting salmon and steelhead for homing (Slatick et al. 1979, 1980; Novotny and Zaugg 1979). The studies were begun with little background knowledge of the effects of disease or certain physiological functions on imprinting and homing in salmonids. Consequently, work aimed at filling this void was begun by the authors in 1978 (Novotny and Zaugg 1979) and continued in 1979. In 1979, we examined random samples of normal populations of homing test fish at the hatcheries to determine the physiological readiness to migrate and adapt to seawater and general fish health. At the Manchester Marine Experimental Station, Manchester, Washington, we determined the survival of samples of the test fish maintained in marine net-pens after release from the hatcheries. Hatcheries and stocks sampled are listed in Table 1. The data collected from random samples were as follows: 1. Physiology. Gill Na+-K+ ATPase . Abnormally low values could be indications that the fish were either in pre- or post-smolt condition, or had been stressed in some way. Plasma electrolytes. Lower than normal values of Na or Cl could indicate immediate problems of osmoregulation when the fish were introduced to seawater; high values may indicate some dehydration due to stress. Increases in levels of K can indicate kidney failure or nitrogen supersaturation stresses
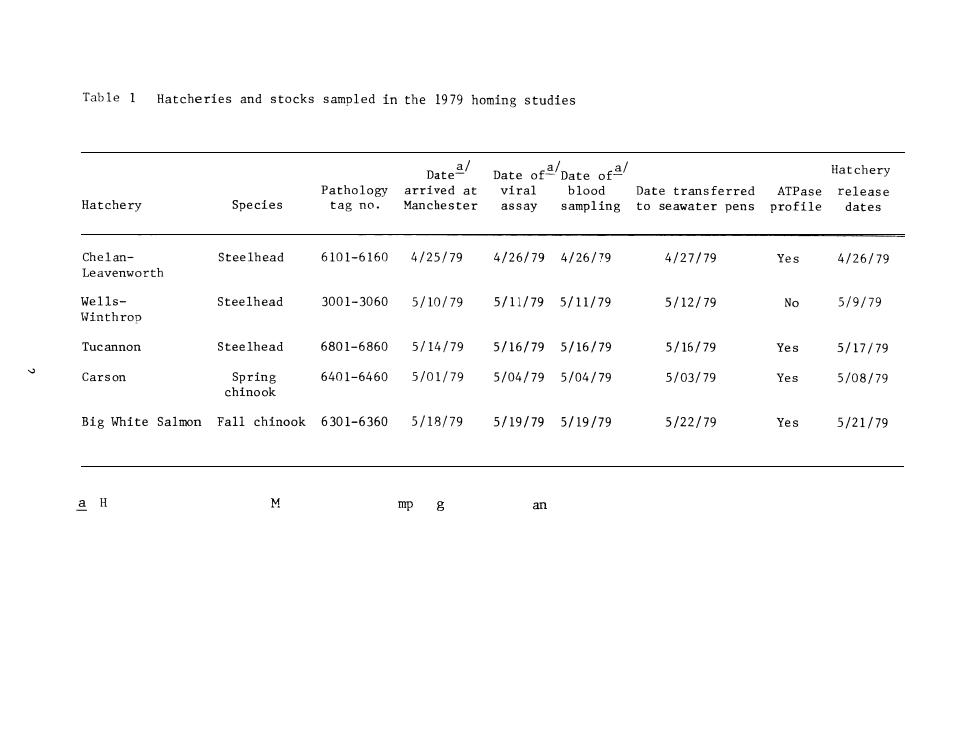
Table 1 Hatcheries and stocks sampled in the 1979 homing studies Hatchery ATPase Hatchery Species tm smeeste Manchester assay sampling to seawater pens profile Chelan- Steelhead 6101-61604/25/794/26/794/26/79 4127179 Yes 4/26/79 Leavenworth Wells- Steelhead 3001-30605/10/795/11/795/11/79 5/12/79 No 5/9/79 Winthrop Tucannon Steelhead 6801-68605/14/79 5/16/795/16/79 5/16/79 Yes 5/17/79 Carson Spring 6401-64605/01/79 5/04/795/04179 5/03/79 Yes 5/08/79 chinook B1gh1teSa1 mon Fa11ch1nook6301-63605/18/795/19/795/19/79 5122/79 Yes 5/21/79 mp g an

Hematocrits and hemoglobins.Values below or above normal ranges usually indicate anemia or dehydration,which can reflect nutritional disease or physiological changes. 2.Fish health The incidence of diseases during freshwater rearing,as reported in hatchery records describing the treatment of fish were examined. The extent of latent bacterial kidney disease (BKD)as determined by indirect fluorescent antibody technique and the presence (or absence)of certain pathogenic viruses were of particular interest. A histological determination of significant lesions or abnormalities in tissue from the gill,eye,liver,kidney,thyroid,brain,and olfactory sac was undertaken. 3.Survival in seawater net-pens Periodic assessments of survival and growth were made,and the major causes of mortality were determined. These surveys were conducted to provide a documentation of the health and physiological (smolt)condition of the populations of fish involved in the tests,especially at the time of imprinting and release.When the marked adult fish return,the data analyzed from the health and physiology surveys should provide us with information that would indicate any adverse influence on survival.Low survival in the marine net-pens caused by poor health or a low percentage of fish transformed through the smolting stages could bias any attempts to relate returns to imprinting. METHODS AND MATERIALS Hatchery Sampling The sampling of fish from the hatchery stocks for health profiles was based on a combination of statistics and economics.Random sampling from 3
Hematocrits and hemoglobins. Values below or above normal ranges usually indicate anemia or dehydration, which can reflect nutritional disease or physiological changes. 2. Fish health The incidence of diseases during freshwater rearing, as reported in hatchery records describing the treatment of fish were examined. The extent of latent bacterial kidney disease (BKD) as determined by indirect fluorescent antibody technique and the presence (or absence) of certain pathogenic viruses were of particular interest. A histological determination of significant lesions or abnormalities in tissue from the gill, eye, liver, kidney, thyroid, brain, and olfactory sac was undertaken. 3. Survival in seawater net-pens Periodic assessments of survival and growth were made, and the major causes of mortality were determined. These surveys were conducted to provide a documentation of the health and physiological (smolt) condition of the populations of fish involved in the tests, especially at the time of imprinting and release. When the marked adult fish return, the data analyzed from the health and physiology surveys should provide us with information that would indicate any adverse influence on survival. Low survival in the marine net-pens caused by poor health or a low percentage of fish transformed through the smolting stages could bias any attempts to relate returns to imprinting. METHODS AND MATERIALS Hatchery Sampling The sampling of fish from the hatchery stocks for health profiles was based on a combination of statistics and economics. Random sampling from 3

populations ranging as high as 100,000 or more showed that a population with a disease incidence of 58 or greater can be detected from a sample of 60 fish (Ossiander and Wedemeyer 1973).Health survey samples of 60 fish were taken at the hatcheries,and held in circular tanks in fresh water at Manchester.In most cases,the tissue and blood samples were collected within 24 hours after arrival at Manchester. Sampling for Physiology Plasma Electrolytes Sodium,potassium,and chloride ion levels in plasma were determined for the Wells Dam Hatchery steelhead,Salmo gairdneri,and Big Creek Hatchery fall chinook salmon,Oncorhynchus tshawytscha,near the time of release.Profiles of plasma electrolytes of the Tucannon and Chelan Hatchery steelhead and Carson Hatchery spring chinook salmon were determined in fresh water and during seawater culture at Manchester. Plasma sodium and potassium values were determined by atomic absorption spectrometry and chlorides with a chloridometer. Gill Na+-K+ATPase During 1979,selected stocks of fall and spring chinook salmon and steelhead trout being reared for release at state and federal hatcheries in the Columbia River drainage were monitored for changes in gill Na+-K+ ATPase to evaluate the state of smoltification at release From tagged releases,we determined the relationship between the state of smoltification at release and length of migration time from the hatchery to the estuary. At approximately 2-week intervals during the spring and summer of 1979,30 fish were removed by dip net from representative ponds or raceways at Tucannon,Carson,Leavenworth (Chelan),and Big White Salmon Hatcheries
populations ranging as high as 100,000 or more showed that a population with a disease incidence of 5% or greater can be detected from a sample of 60 fish (Ossiander and Wedemeyer 1973). Health survey samples of 60 fish were taken at the hatcheries, and held in circular tanks in fresh water at Manchester. In most cases, the tissue and blood samples were collected within 24 hours after arrival at Manchester. Sampling for Physiology Plasma Electrolytes Sodium, potassium, and chloride ion levels in plasma were determined for the Wells Dam Hatchery steelhead, Salmo gairdneri, and Big Creek Hatchery fall chinook salmon, Oncorhynchus tshawytscha, near the time of release. Profiles of plasma electrolytes of the Tucannon and Chelan Hatchery steelhead and Carson Hatchery spring chinook salmon were determined in fresh water and during seawater culture at Manchester. Plasma sodium and potassium values were determined by atomic absorption spectrometry and chlorides with a chloridometer. Gill Na+-K+ ATPase During 1979, selected stocks of fall and spring chinook salmon and steelhead trout being reared for release at state and federal hatcheries in the Columbia River drainage were monitored for changes in gill Na+-K+ ATPase to evaluate the state of smoltification at release. From tagged releases, we determined the relationship between the state of smoltification at release and length of migration time from the hatchery to the estuary. At approximately 2-week intervals during the spring and summer of 1979, 30 fish were removed by dip net from representative ponds or raceways at Tucannon, Carson, Leavenworth (Chelan), and Big White Salmon Hatcheries. 4

Steelhead from the Wells Dam rearing ponds were sampled only at release. Ten groups of three fish each were anesthestized or killed by a blow on the head at each sampling.After weights and/or fork lengths were determined, approximately equal quantities of gill filaments were removed from the gill arches of each of the three fish in the group (total weight of gill filaments-0.1 to 0.2 g)and processed as described in our previous report (Novotny and Zaugg 1979). Disease Sampling Life History of Hatchery Juveniles Husbandry techniques,disease,and environmental history may have deleterious effects on fish health and smolt quality (Wedemeyer et al. 1979;Folmar and Dickhoff 1979).Many chemotherapeutic compounds used in the treatment of parasitic and bacterial diseases of fish may affect smoltification (Lorz and McPherson 1976),and subclinical infections may be exacerbated by the stress of seawater entry. The information (Table 2)was obtained from hatchery management and is self-explanatory.Where information was not obtained,the entries have been left blank. Blood Sample Collection The fish were lightly anesthetized in an aerated 1:20,000 solution of MS-222.In the larger fish,blood was sampled from the caudal arch with a 1 cc heparinized syringe and a 25 gauge hypodermic needle.Small fish were bled by severing the caudal peduncle and collecting the blood in heparinized capillary tubes. Blood samples taken for hematocrits (packed cell volume)were
Steelhead from the Wells Dam rearing ponds were sampled only at release. Ten groups of three fish each were anesthestized or killed by a blow on the head at each sampling. After weights and/or fork lengths were determined, approximately equal quantities of gill filaments were removed from the gill arches of each of the three fish in the group (total weight of gill filaments-O.1 to 0.2 g) and processed as described in our previous report (Novotny and Zaugg 1979). Disease Sampling Life History of Hatchery Juveniles Husbandry techniques, disease, and environmental history may have deleterious effects on fish health and smolt quality (Wedemeyer et al. 1979; Folmar and Dickhoff 1979). Many chemotherapeutic compounds used in the treatment of parasitic and bacterial diseases of fish may affect smoltification (Lorz and McPherson 1976), and subclinical infections may be exacerbated by the stress of seawater entry. The information (Table 2) was obtained from hatchery management and is self-explanatory. Where information was not obtained, the entries have been left blank. Blood Sample Collection The fish were lightly anesthetized in an aerated 1:20,000 solution of MS-222. In the larger fish, blood was sampled from the caudal arch with a 1 cc heparinized syringe and a 25 gauge hypodermic needle. Small fish were bled by severing the caudal peduncle and collecting the blood in heparinized capillary tubes. Blood samples taken for hematocrits (packed cell volume) were
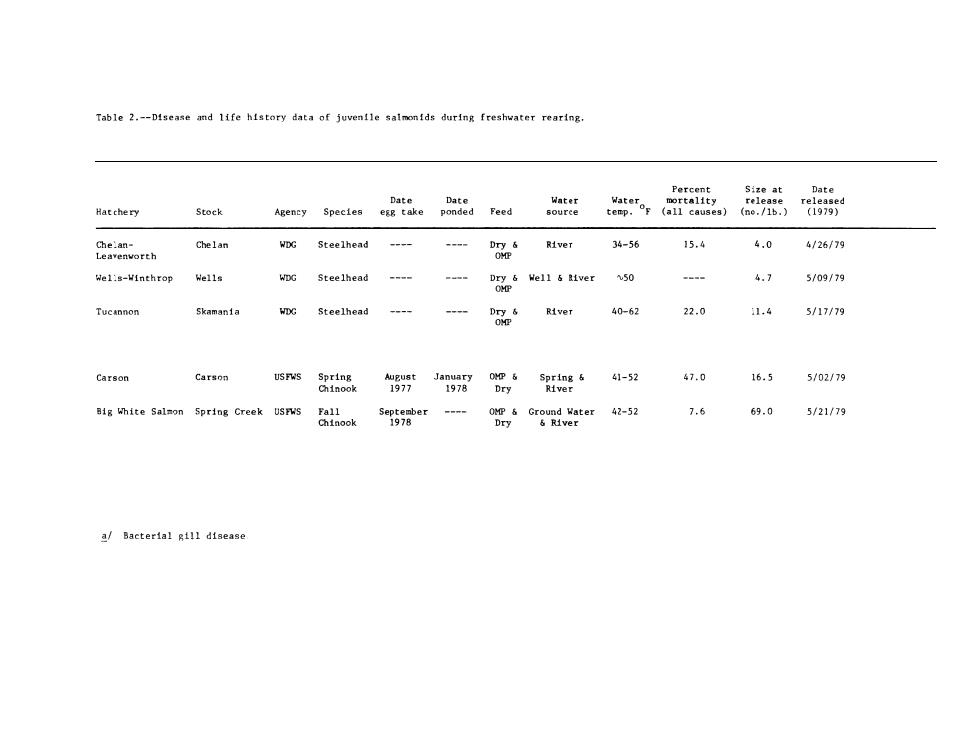
Table 2.--Disease and life history data of juventle salmonids during freshvater rearing. Hatchery seer secte red Chgimiorth Gelan vc steeihead tver 34-56 15.44.04/26179 wel:s-Vinthrop Mells WDG Steelhead 4.75109/79 Tucannon Skamania VG Steelhead 22.0 1.4 5/17179 anns3:‘-g06;0m tig mite salmon ordne Creek esnsrger 7.669.05/21/79