
Classes of Antibodies Since antibodies have a high molecular weight they make very good antigens and we can produce antibodies against another antibody.Differences in amino acid sequences between antibody classes,subclasses,and types can be determined by antigenic specificity of antibodies.Antibodies carry three major categories of antigenic determinants:isotypic,allotypic,and idiotypic Isotypic Determinants These determinants distinguish the C regions of the H chain classes (and subclasses) and the L chain types.Each isotype is encoded by a distinct gene that is characteristic for a particular mammalian species and is present in all members of that species.Antibodies to these determinants may be raised by injecting IgG from one spe ies intoanother species.Each I class (IgG.IgM,IgA,IgD,and IgE)possesses a specific isotypic determinant on its H chain. Allotypic Determinants The second group of antigenic determinants are those found on the Igs of some,but not all,members of a particular species.Allotypic determinants reflect genetic the alloantigen.In human the Cm antigens are a good example. Idiotypic Determinants The third group of antigenic determinants of Igs exist as a result of unique structures generated by the hypervariable subregions (or complementarity-determinging regions CDR)on L and H chains.The antigenic determinants are called idiotopes,analogous to epitopes of classical antigens. Alpha-idiotopes lie outside the Ag-binding site. Beta-idiotopes are close to the antigen binding site. Gamma-idiotopes are formed by the antigen binding site. Antiidiotypic antibodies may be formed in self naturally or induced artificially in self.The significance of these molecules relates to their importance for immune regulation
Classes of Antibodies Since antibodies have a high molecular weight they make very good antigens and we can produce antibodies against another antibody. Differences in amino acid sequences between antibody classes, subclasses, and types can be determined by antigenic specificity of antibodies. Antibodies carry three major categories of antigenic determinants: isotypic, allotypic, and idiotypic. Isotypic Determinants These determinants distinguish the C regions of the H chain classes (and subclasses) and the L chain types. Each isotype is encoded by a distinct gene that is characteristic for a particular mammalian species and is present in all members of that species. Antibodies to these determinants may be raised by injecting IgG from one species into another species. Each Ig class (IgG. IgM, IgA, IgD, and IgE) possesses a specific isotypic determinant on its H chain. Allotypic Determinants The second group of antigenic determinants are those found on the Igs of some, but not all, members of a particular species. Allotypic determinants reflect genetic polymorphism of Igs within one species. Antibodies can be formed against an allotypic determinant by injecting Igs into another member of the species that does not possess the alloantigen. In human the Gm antigens are a good example. Idiotypic Determinants The third group of antigenic determinants of Igs exist as a result of unique structures generated by the hypervariable subregions (or complementarity-determinging regions CDR) on L and H chains. The antigenic determinants are called idiotopes, analogous to epitopes of classical antigens. Alpha-idiotopes lie outside the Ag-binding site. Beta-idiotopes are close to the antigen binding site. Gamma-idiotopes are formed by the antigen binding site. Antiidiotypic antibodies may be formed in self naturally or induced artificially in self. The significance of these molecules relates to their importance for immune regulation
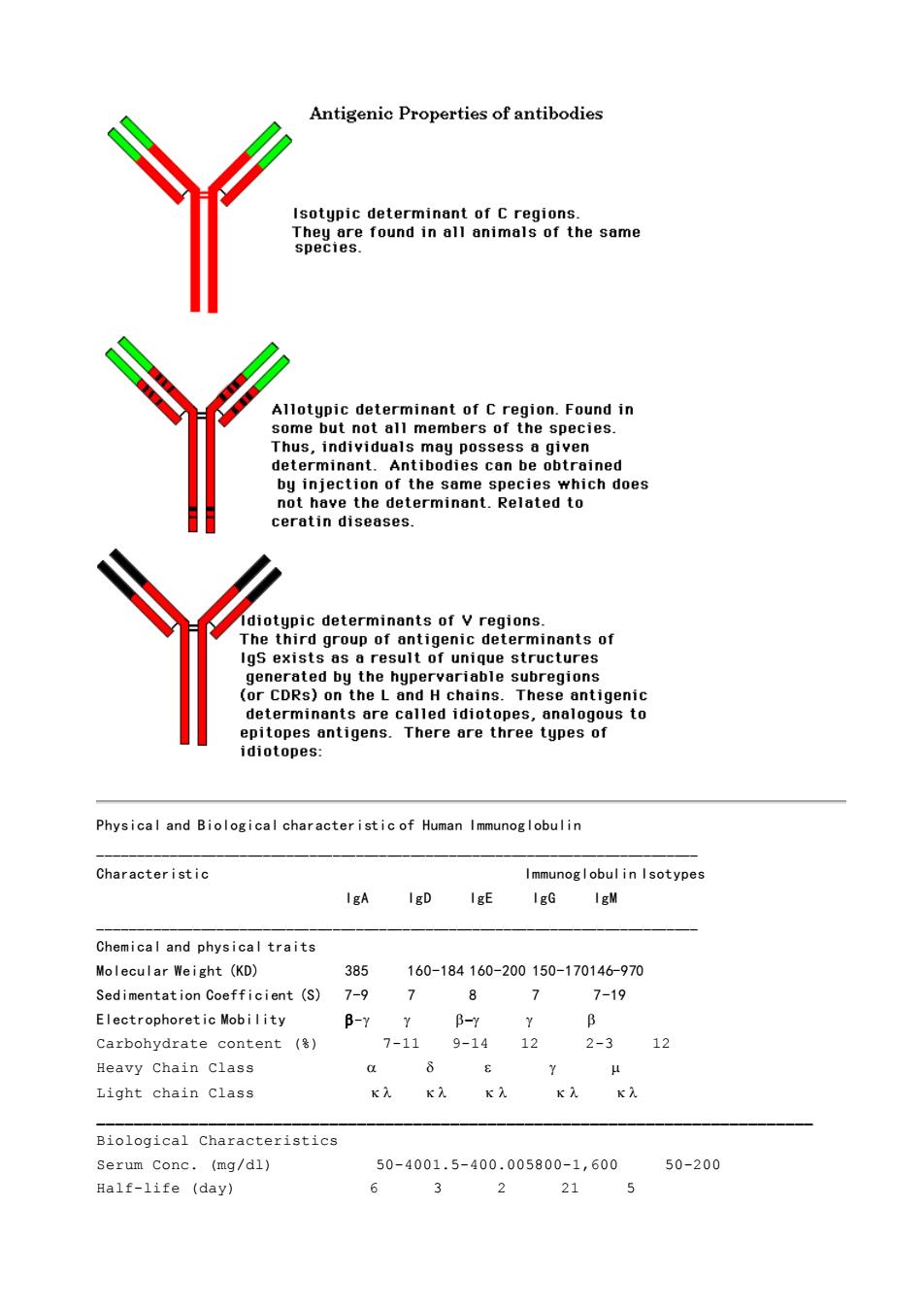
Antigenic properties of antibodies Isotypic determinant of C regions. eround in mof thesme Allotypic determinant of C region.Found in Thus,individuals may po determinant.Antibodies can be obtrained ich does ceratin diseases. otypic determinants of v regions. irdg9r8oe8nt1geneemiCautego generated by the hypervariable subregions CDRs)on the santigens.There are three tugus to e ca diotopes Physical and Biological characteristic of Human Immunoglobulin Characteristic mmunogl obul in Isotypes IgA IgD IgE IgG IgM Chemical and physical traits cular Weight (KD) 385 160-184160-200150-170146-970 Sedimentation Coefficient(S) 7-9 8 7 719 Electrophoretic Mobility B-Y y B-Y y Carbohydrate content() 7-119-1412 2-312 Heavy chain class 8 Light chain class x) Biological Characteristics Serum Conc.(mg/dl) 50-4001.5-400.005800-1,600 50-200 Half-life (day) 6 3 2 21
Physical and Biological characteristic of Human Immunoglobulin _____________________________________________________________________________ Characteristic Immunoglobulin Isotypes IgA IgD IgE IgG IgM _____________________________________________________________________________ Chemical and physical traits Molecular Weight (KD) 385 160-184 160-200 150-170146-970 Sedimentation Coefficient (S) 7-9 7 8 7 7-19 Electrophoretic Mobility - − Carbohydrate content (%) 7-11 9-14 12 2-3 12 Heavy Chain Class Light chain Class _____________________________________________________________________________ Biological Characteristics Serum Conc. (mg/dl) 50-4001.5-400.005800-1,600 50-200 Half-life (day) 6 3 2 21 5
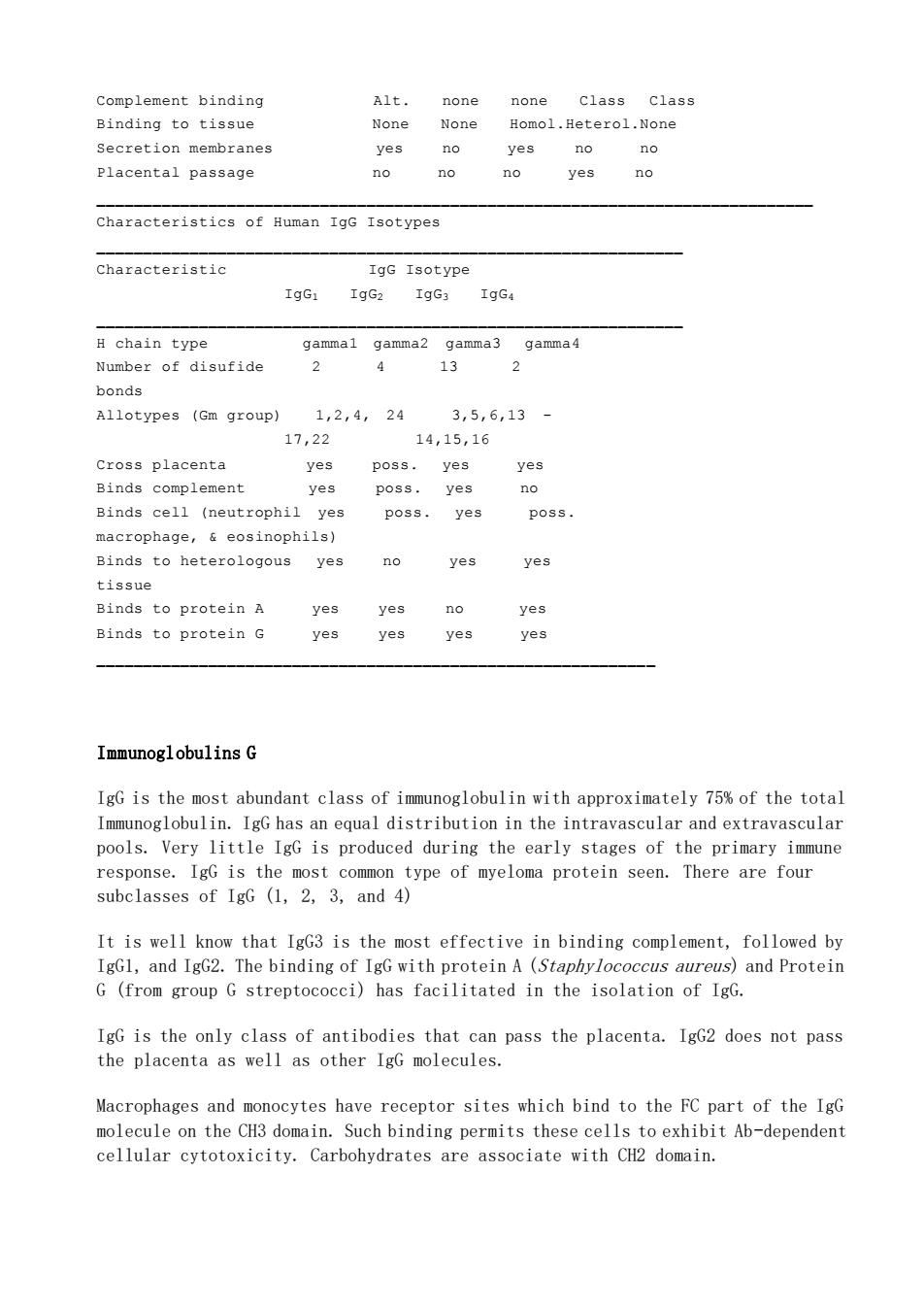
Complement binding Alt. none none class class Binding to tissue None None Homol.Heterol.None Secretion membranes s no ves no no Placental passage no no no yes no Characteristics of Human IgG Isotypes Characteristic IgG Isotype IgG: H chain type gammal gamma2 gamma3 gamma4 Number of disufide 2 4 13 2 bonds Allotypes (Gm group) 1,2,4,24 3,5,6,13- 17,22 14,15,16 Cross placenta yes poss.yes yes Binds complement yes poss.yes no Binds cell (neutrophil yes poss.yes macrophage,eosinophils) Binds to heterologous yes no yes yes tissue Binds to protein A yes yes no yes Binds to protein G yes yes yes yes Immunoglobulins G IgG is the most abundant class of immunoglobulin with approximately 75%of the total Immunoglobulin.IgG has an equal distribution in the intravascular and extravascular pools.Very little IgG is produced during the early stages of the primary immune response.IgG is the common type of myeloma protein seen.There are four subclasses of IgG (1,2.3,and 4) It is well know that IgG3 is the most effective in binding complement,followed by IgGl,and IgG2.The binding of IgG with protein A (Staphylococcus aureus)and Protein G(from group G streptococci)has facilitated in the isolation of IgG. IgG is the only class of antibodies that can pass the placenta.IgG2 does not pass the placenta as well as other IgG molecules. Macrophages and monocytes have receptor sites which bind to the FC part of the IgG molecule on the CH3 domain.Such binding permits these cells to exhibit Ab-dependent cellular cytotoxicity.Carbohydrates are associate with CH2 domain
Complement binding Alt. none none Class Class Binding to tissue None None Homol.Heterol.None Secretion membranes yes no yes no no Placental passage no no no yes no _____________________________________________________________________________ Characteristics of Human IgG Isotypes _______________________________________________________________ Characteristic IgG Isotype IgG1 IgG2 IgG3 IgG4 _______________________________________________________________ H chain type gamma1 gamma2 gamma3 gamma4 Number of disufide 2 4 13 2 bonds Allotypes (Gm group) 1,2,4, 24 3,5,6,13 - 17,22 14,15,16 Cross placenta yes poss. yes yes Binds complement yes poss. yes no Binds cell (neutrophil yes poss. yes poss. macrophage, & eosinophils) Binds to heterologous yes no yes yes tissue Binds to protein A yes yes no yes Binds to protein G yes yes yes yes ____________________________________________________________ Immunoglobulins G IgG is the most abundant class of immunoglobulin with approximately 75% of the total Immunoglobulin. IgG has an equal distribution in the intravascular and extravascular pools. Very little IgG is produced during the early stages of the primary immune response. IgG is the most common type of myeloma protein seen. There are four subclasses of IgG (1, 2, 3, and 4) It is well know that IgG3 is the most effective in binding complement, followed by IgG1, and IgG2. The binding of IgG with protein A (Staphylococcus aureus) and Protein G (from group G streptococci) has facilitated in the isolation of IgG. IgG is the only class of antibodies that can pass the placenta. IgG2 does not pass the placenta as well as other IgG molecules. Macrophages and monocytes have receptor sites which bind to the FC part of the IgG molecule on the CH3 domain. Such binding permits these cells to exhibit Ab-dependent cellular cytotoxicity. Carbohydrates are associate with CH2 domain
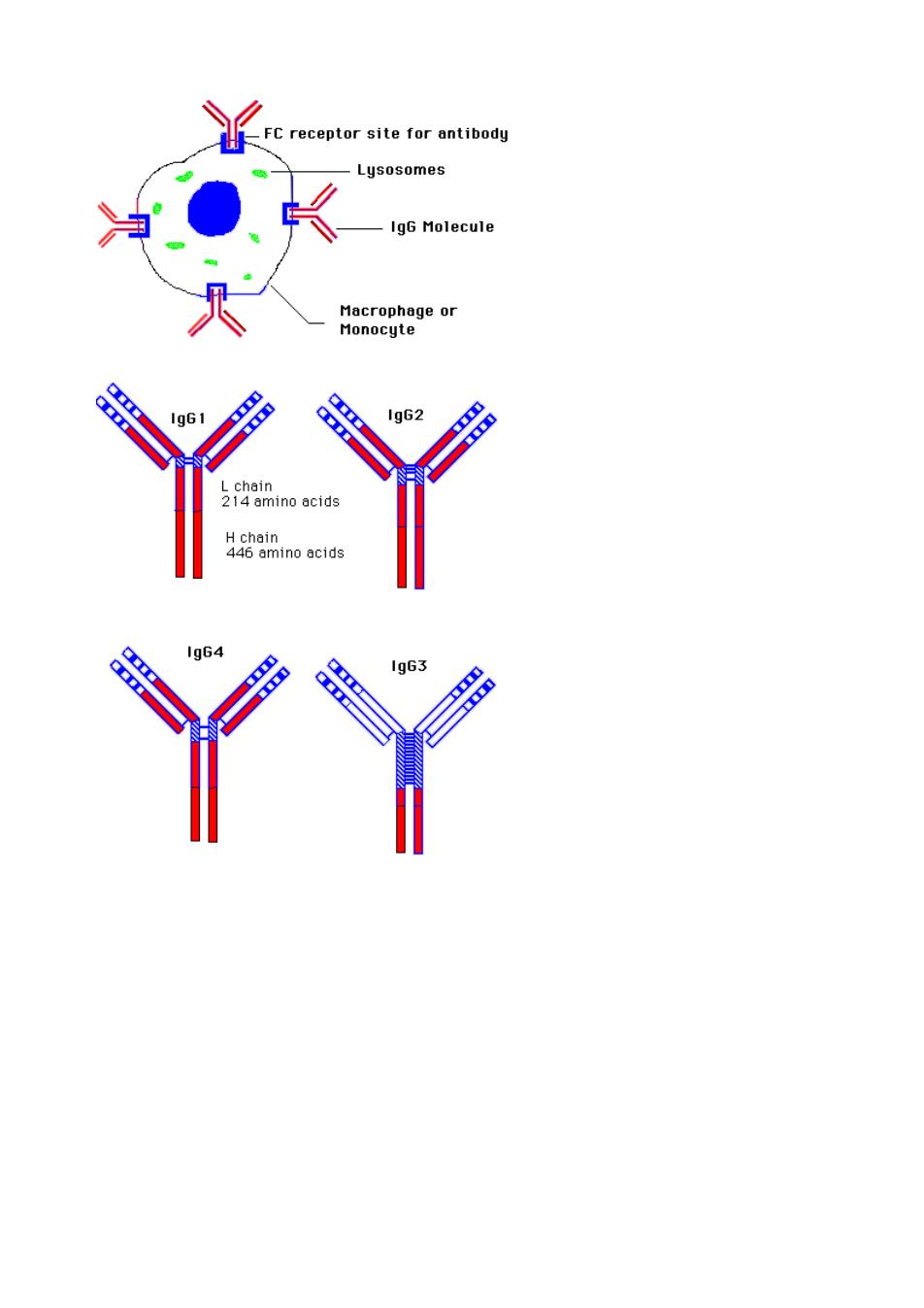
-FC receptor site for antibody Lusosomes IgG Molecule Macrophage or Monocyte g62
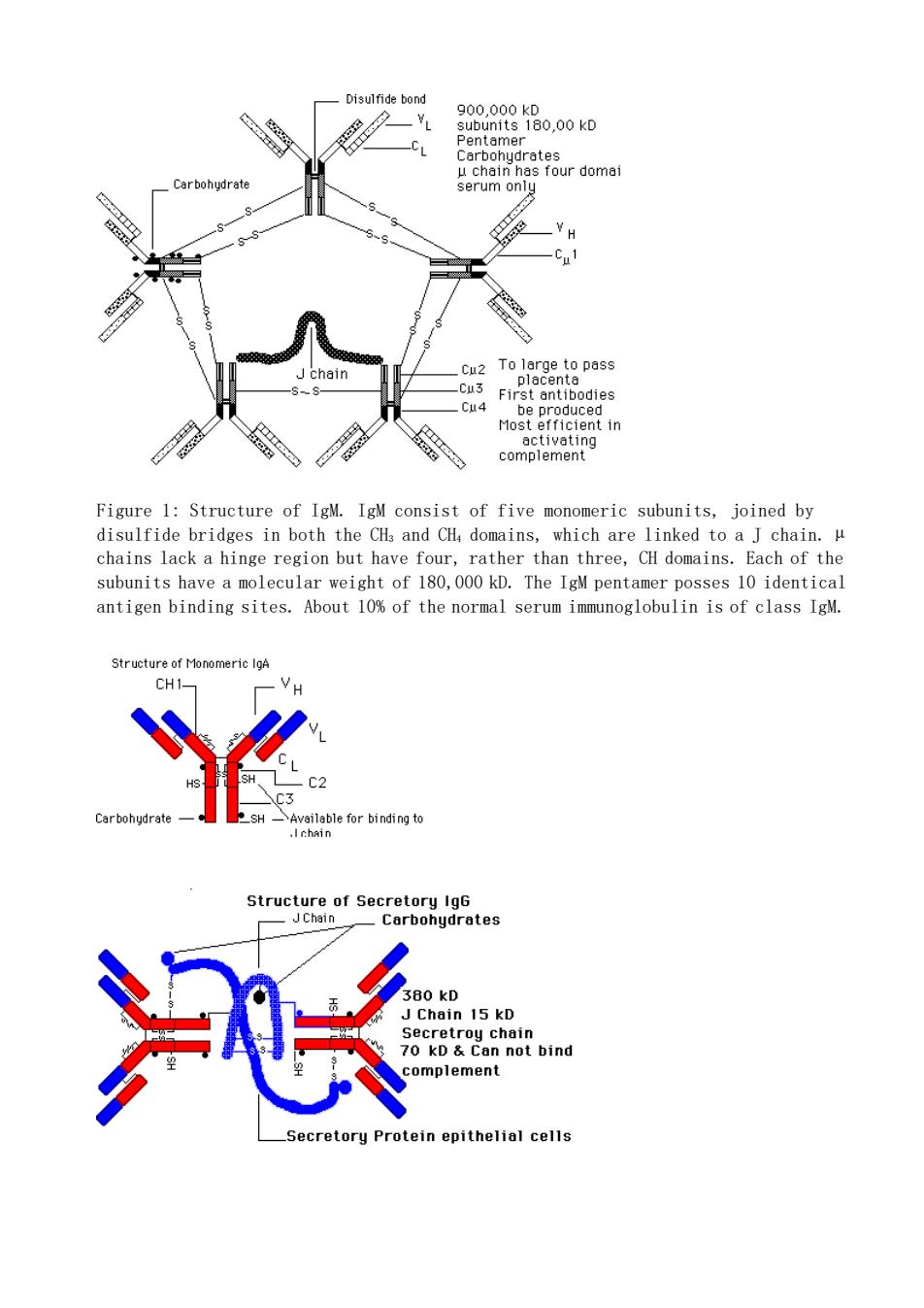
Disulfide bond erum on 8 Cu2 Cu Most efficient in Figure 1:Structure of IgM.IgM consist of five monomeric subunits,joined by disulfide bridges in both the CHa and CH domains,which are linked to a J chain. chains lack a hinge region but have four,rather than three,CH domains.Each of the subunits have a molecular weight of 180,000 kD.The IgM pentamer posses 10 identical antigen binding sites.About 10%of the normal serum immunoglobulin is of class IgM Structure of Secretory IgG -Carbohydrates 15 kD an not ecretoru protein epithelial cells
Figure 1: Structure of IgM. IgM consist of five monomeric subunits, joined by disulfide bridges in both the CH3 and CH4 domains, which are linked to a J chain. µ chains lack a hinge region but have four, rather than three, CH domains. Each of the subunits have a molecular weight of 180,000 kD. The IgM pentamer posses 10 identical antigen binding sites. About 10% of the normal serum immunoglobulin is of class IgM

Figure 2:Structure of monomeric and secretory IgA antibody.The dimeric form is jointed by the J chain linked to FC region (CH,)by disulfide bonds.IgA is about 15%of human serum immunoglobulin.In the serum it is in the monomeric form.The most important form is in the dimeric form known as secretory IgG.It is found in milk tears,nasal fluid,saliva,perspiration,genitourinary secretion and secretion of the lungs and intestine.Its function is to protect epithelial surfaces from pathogens. Secretory IgA is resistant to protein digestion. FIGURE 3:Structure of lgD igure 4:Structure of IgE Carbohydrate ss the plecenta 160-200kD u2 site for att ment to mess cella Return This page is maintained by the Natural Toxins Research Center at Texas A&M University Kingsville
Figure 2: Structure of monomeric and secretory IgA antibody. The dimeric form is jointed by the J chain linked to FC region (CH3) by disulfide bonds. IgA is about 15% of human serum immunoglobulin. In the serum it is in the monomeric form. The most important form is in the dimeric form known as secretory IgG. It is found in milk, tears, nasal fluid, saliva, perspiration, genitourinary secretion and secretion of the lungs and intestine. Its function is to protect epithelial surfaces from pathogens. Secretory IgA is resistant to protein digestion. Return This page is maintained by the Natural Toxins Research Center at Texas A&M University - Kingsville