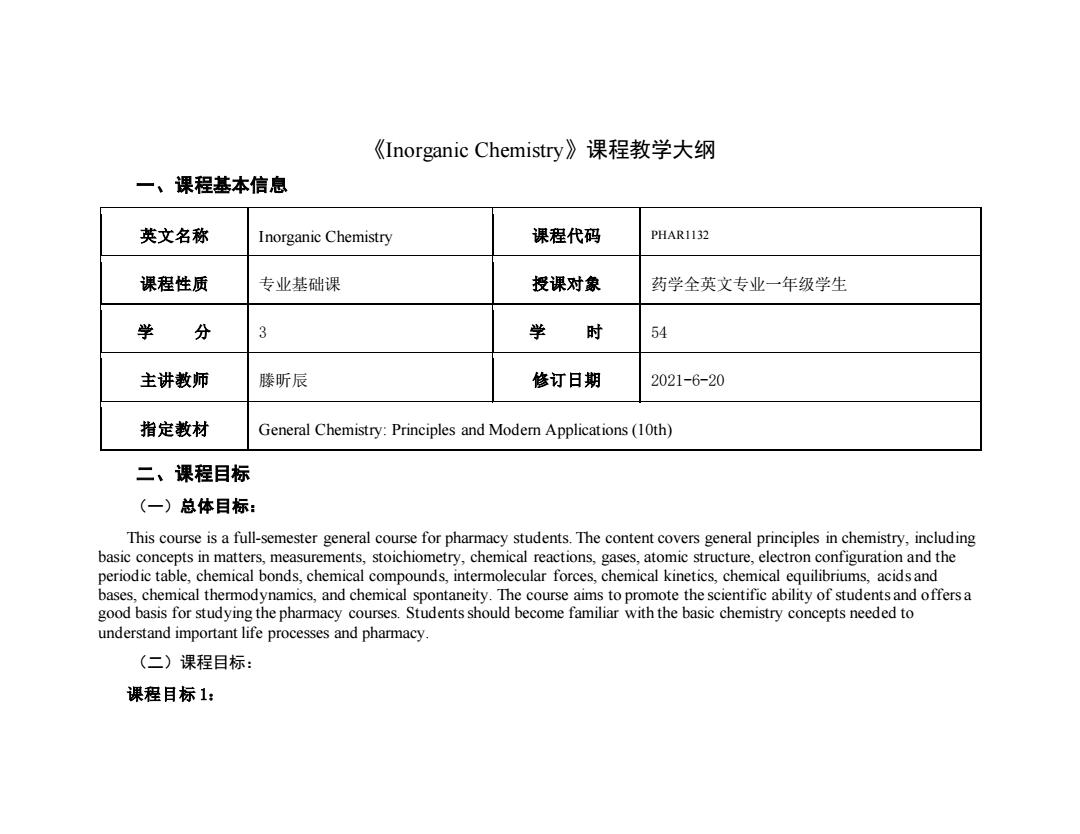
《Inorganic Chemistry》课程教学大纲 一、课程基本信息 英文名称 Inorganic Chemistry 课程代码 PHAR1132 课程性质 专业基础课 授课对象 药学全英文专业一年级学生 学 分 3 学 时 54 主讲教师 滕昕辰 修订日期 2021-6-20 指定教材 General Chemistry:Principles and Modern Applications(10th) 二、课程目标 (一)总体目标: This course is a full-semester general course for pharmacy students.The content covers general principles in chemistry,including basic concepts in matters,measurements,stoichiometry,chemical reactions,gases,atomic structure,electron configuration and the periodic table,chemical bonds,chemical compounds,intermolecular forces,chemical kinetics,chemical equilibriums,acids and bases,chemical thermodynamics,and chemical spontaneity.The course aims to promote the scientific ability of students and offersa good basis for studying the pharmacy courses.Students should become familiar with the basic chemistry concepts needed to understand important life processes and pharmacy. (二)课程目标: 课程目标1:
《Inorganic Chemistry》课程教学大纲 一、课程基本信息 英文名称 Inorganic Chemistry 课程代码 PHAR1132 课程性质 专业基础课 授课对象 药学全英文专业一年级学生 学 分 3 学 时 54 主讲教师 滕昕辰 修订日期 2021-6-20 指定教材 General Chemistry: Principles and Modern Applications (10th) 二、课程目标 (一)总体目标: This course is a full-semester general course for pharmacy students. The content covers general principles in chemistry, including basic concepts in matters, measurements, stoichiometry, chemical reactions, gases, atomic structure, electron configuration and the periodic table, chemical bonds, chemical compounds, intermolecular forces, chemical kinetics, chemical equilibriums, acids and bases, chemical thermodynamics, and chemical spontaneity. The course aims to promote the scientific ability of students and offers a good basis for studying the pharmacy courses. Students should become familiar with the basic chemistry concepts needed to understand important life processes and pharmacy. (二)课程目标: 课程目标 1:
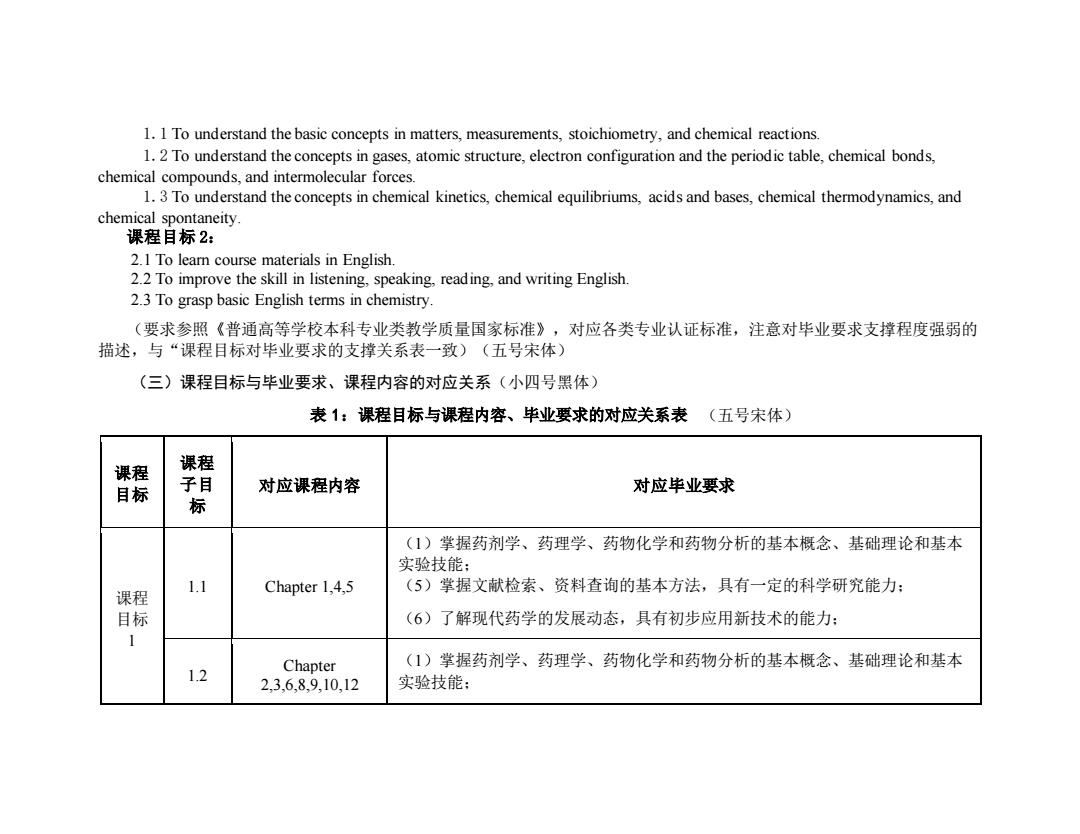
1.1 To understand the basic concepts in matters,measurements,stoichiometry,and chemical reactions. 1.2 To understand the concepts in gases,atomic structure,electron configuration and the periodic table,chemical bonds, chemical compounds,and intermolecular forces. 1.3 To understand the concepts in chemical kinetics,chemical equilibriums,acids and bases,chemical thermodynamics,and chemical spontaneity. 课程目标2: 2.1 To learn course materials in English. 2.2 To improve the skill in listening,speaking,reading,and writing English. 2.3 To grasp basic English terms in chemistry. (要求参照《普通高等学校本科专业类教学质量国家标准》,对应各类专业认证标准,注意对毕业要求支撑程度强弱的 描述,与“课程目标对毕业要求的支撑关系表一致)(五号宋体) (三)课程目标与毕业要求、课程内容的对应关系(小四号黑体) 表1:课程目标与课程内容、毕业要求的对应关系表(五号宋体) 课程 课程 目标 子目 对应课程内容 对应毕业要求 标 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能: 1.1 Chapter 1,4,5 (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力: 课程 目标 (6)了解现代药学的发展动态,具有初步应用新技术的能力: 1 Chapter (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 1.2 23.6,89,10,12 实验技能:
1.1 To understand the basic concepts in matters, measurements, stoichiometry, and chemical reactions. 1.2 To understand the concepts in gases, atomic structure, electron configuration and the periodic table, chemical bonds, chemical compounds, and intermolecular forces. 1.3 To understand the concepts in chemical kinetics, chemical equilibriums, acids and bases, chemical thermodynamics, and chemical spontaneity. 课程目标 2: 2.1 To learn course materials in English. 2.2 To improve the skill in listening, speaking, reading, and writing English. 2.3 To grasp basic English terms in chemistry. (要求参照《普通高等学校本科专业类教学质量国家标准》,对应各类专业认证标准,注意对毕业要求支撑程度强弱的 描述,与“课程目标对毕业要求的支撑关系表一致)(五号宋体) (三)课程目标与毕业要求、课程内容的对应关系(小四号黑体) 表 1:课程目标与课程内容、毕业要求的对应关系表 (五号宋体) 课程 目标 课程 子目 标 对应课程内容 对应毕业要求 课程 目标 1 1.1 Chapter 1,4,5 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能; (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力; (6)了解现代药学的发展动态,具有初步应用新技术的能力; 1.2 Chapter 2,3,6,8,9,10,12 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能;
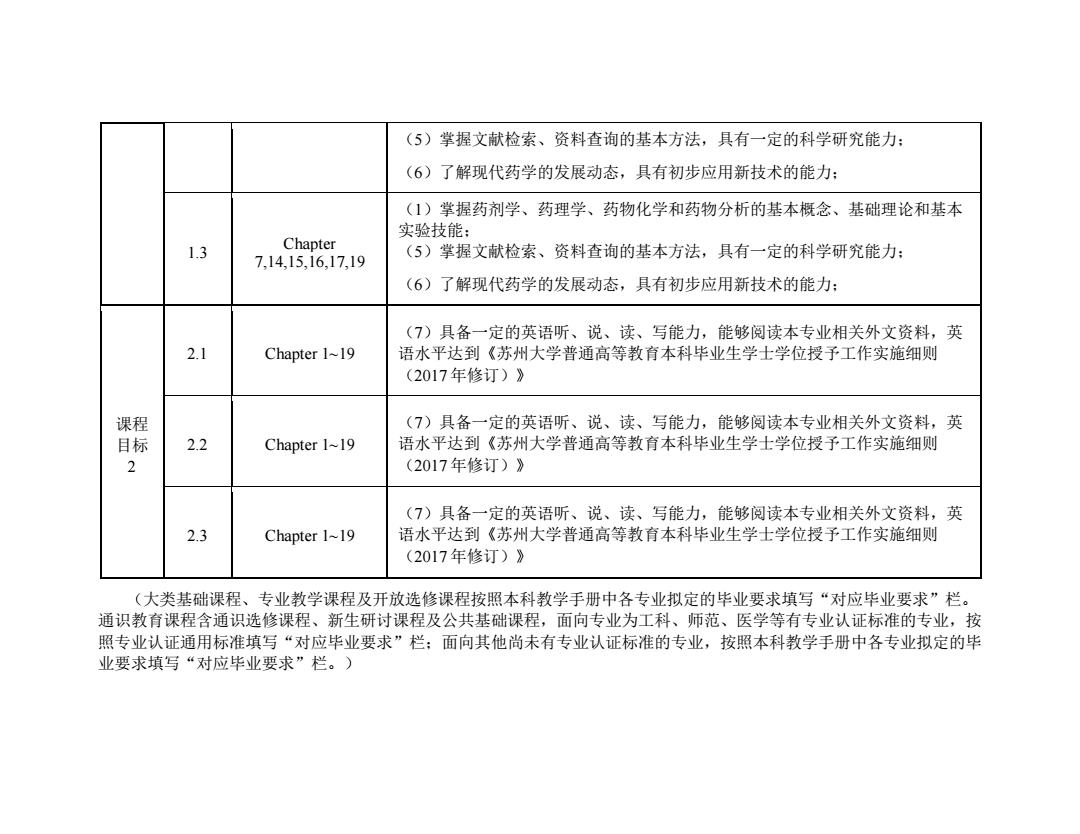
(5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力: (6)了解现代药学的发展动态,具有初步应用新技术的能力: (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能: 1.3 Chapter (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力: 7,14,15,16,17,19 (6)了解现代药学的发展动态,具有初步应用新技术的能力: (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 2.1 Chapter 1~19 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017年修订)》 课程 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 目标 2.2 Chapter 1~19 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 2 (2017年修订)》 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 2.3 Chapter 1~19 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017年修订)》 (大类基础课程、专业教学课程及开放选修课程按照本科教学手册中各专业拟定的毕业要求填写“对应毕业要求”栏。 通识教育课程含通识选修课程、新生研讨课程及公共基础课程,面向专业为工科、师范、医学等有专业认证标准的专业,按 照专业认证通用标准填写“对应毕业要求”栏:面向其他尚未有专业认证标准的专业,按照本科教学手册中各专业拟定的毕 业要求填写“对应毕业要求”栏。)
(5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力; (6)了解现代药学的发展动态,具有初步应用新技术的能力; 1.3 Chapter 7,14,15,16,17,19 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能; (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力; (6)了解现代药学的发展动态,具有初步应用新技术的能力; 课程 目标 2 2.1 Chapter 1~19 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017 年修订)》 2.2 Chapter 1~19 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017 年修订)》 2.3 Chapter 1~19 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017 年修订)》 (大类基础课程、专业教学课程及开放选修课程按照本科教学手册中各专业拟定的毕业要求填写“对应毕业要求”栏。 通识教育课程含通识选修课程、新生研讨课程及公共基础课程,面向专业为工科、师范、医学等有专业认证标准的专业,按 照专业认证通用标准填写“对应毕业要求”栏;面向其他尚未有专业认证标准的专业,按照本科教学手册中各专业拟定的毕 业要求填写“对应毕业要求”栏。)

三、教学内容 Chapter 1.Matter:Its Properties and Measurement 1.教学目标 Learn how to use the scientific methods to study a subject Be able to classify matters ● Understand the general properties of different matters Know how to make measurements properly 2. 教学重难点 教学重点: Definition,properties and classification of matters Measurement ofmatters 教学难点 Classification of matters Unit conversion Uncertainties in scientific measurements Significant figures in calculations 3. 教学内容 1. The Scientific Method How to study chemistry The definition of hypothesis,law and theory 2.Properties of Matter Physical properties and physical changes Chemical properties and chemical changes Extensive and intensive properties 3.Classification of Matter Identification of matter Matter at the atomic level 4.Measurement of Matter:SI(Metric)Units
三、教学内容 Chapter 1. Matter: Its Properties and Measurement 1. 教学目标 • Learn how to use the scientific methods to study a subject • Be able to classify matters • Understand the general properties of different matters • Know how to make measurements properly 2. 教学重难点 教学重点: • Definition, properties and classification of matters • Measurement of matters 教学难点: • Classification of matters • Unit conversion • Uncertainties in scientific measurements • Significant figures in calculations 3. 教学内容 1. The Scientific Method How to study chemistry The definition of hypothesis, law and theory 2. Properties of Matter Physical properties and physical changes Chemical properties and chemical changes Extensive and intensive properties 3. Classification of Matter Identification of matter Matter at the atomic level 4. Measurement of Matter: SI (Metric) Units

Measurements are quantitative observations SI base units and their definition Derived SI units Rules of using SI units Converting units 5.Density and Percent Composition:Their Use in Problem Solving Measuring and calculating density Calculating percent composition 6.Uncertainties in Scientific Measurements Definition of uncertainty Precision and accuracy 1-7 Significant Figures Definition of significant figures Significant figures in calculations 4. 教学方法 多媒体教学: Illustration ofscientific methods Illustration ofclassification of matters Illustration ofmatterat microscopic level ● Illustration ofdifferent types of measurements Illustration ofsignificant figure in calculations 互动教学: What kind of matter is...(air,oxygen,tap water,distilled water,soda,woods)? Why need to standardize units? How to achieve precision and accuracy? How to determine uncertainty in measurement? How to maintain the precision of measurement during calculation? 5. 教学评价 课后问题:
Measurements are quantitative observations SI base units and their definition Derived SI units Rules of using SI units Converting units 5. Density and Percent Composition: Their Use in Problem Solving Measuring and calculating density Calculating percent composition 6. Uncertainties in Scientific Measurements Definition of uncertainty Precision and accuracy 1-7 Significant Figures Definition of significant figures Significant figures in calculations 4. 教学方法 多媒体教学: • Illustration of scientific methods • Illustration of classification of matters • Illustration of matter at microscopic level • Illustration of different types of measurements • Illustration of significant figure in calculations 互动教学: • What kind of matter is … (air, oxygen, tap water, distilled water, soda, woods)? • Why need to standardize units? • How to achieve precision and accuracy? • How to determine uncertainty in measurement? • How to maintain the precision of measurement during calculation? 5. 教学评价 课后问题:

Textbook,Chapter1:7,10,12,23,31,32,54,61,69,89 Chapter 2.Atoms and the Atomic Theory 1.教学目标 Understand the structure of atoms Understand the general properties ofatoms Get a genera idea about the periodic table Understand the meaning of molar mass 2.教学重难点 教学重点: The historical path leading to identification of atom ic structure Mass number,atomic number,neutron number,and atomic mass of an atom Trends in periods and groups Definition of mole and Avogadro's number 教学难点 Experiments leading to discovery of electrons and nucleus Converting between mass,number of moles,and number of atoms 3.教学内容 2-1 Early Chemical Discoveries and the Atomic Theory Law of conservation of matter Law of constant composition John Dalton's atomic theory Law of multiple proportions 2-2 Electrons and Other Discoveries in Atomic Physics Thomson's cathode-ray experiment and Thomson's model of the atom Millikan's oil drop experiment and determination of the charge and mass of the electron 2-3 The Nuclear Atom Rutherford's gold foil experiment and Rutherford's model of the atom Discovery of protons Discovery of neutrons
• Textbook, Chapter 1: 7, 10, 12, 23, 31, 32, 54, 61, 69, 89 Chapter 2. Atoms and the Atomic Theory 1. 教学目标 • Understand the structure of atoms • Understand the general properties of atoms • Get a genera idea about the periodic table • Understand the meaning of molar mass 2. 教学重难点 教学重点: • The historical path leading to identification of atomic structure • Mass number, atomic number, neutron number, and atomic mass of an atom • Trends in periods and groups • Definition of mole and Avogadro’s number 教学难点: • Experiments leading to discovery of electrons and nucleus • Converting between mass, number of moles, and number of atoms 3. 教学内容 2-1 Early Chemical Discoveries and the Atomic Theory Law of conservation of matter Law of constant composition John Dalton’s atomic theory Law of multiple proportions 2-2 Electrons and Other Discoveries in Atomic Physics Thomson’s cathode-ray experiment and Thomson’s model of the atom Millikan’s oil drop experiment and determination of the charge and mass of the electron 2-3 The Nuclear Atom Rutherford’s gold foil experiment and Rutherford’s model of the atom Discovery of protons Discovery of neutrons

Atomic structure Mass number,atomic number(proton number),and neutron number 2-4 Chemical Elements Chemical symbols Isotopes and the percent natural abundances Mass spectrometer 2-5 Atomic Mass Definition of atomic mass Atomic mass units Average atomic masses 2-6 Introduction to the Periodic Table Types ofelements Trends in periods and groups Introduction of main group elements 2-7 The Concept of the Mole and the Avogadro Constant Definition of mole and Avogadro's number Definition of molar mass 2-8 Using the Mole Concept in Calculations Converting between mass,number of moles,and number of atoms 4. 教学方法 多媒体教学: Illustration of law of conservation of matterand law of multiple proportions Illustration ofkey experiments leading to discoveries in atomic physics Illustration ofisotopes and massspectra Illustration ofperiodic table 互动教学: How can cathod-ray experiment prove the existence of electrons What is the identity of each of the radiation rays? How can the gold-foil experiment tell us the structure of anatom?
Atomic structure Mass number, atomic number (proton number), and neutron number 2-4 Chemical Elements Chemical symbols Isotopes and the percent natural abundances Mass spectrometer 2-5 Atomic Mass Definition of atomic mass Atomic mass units Average atomic masses 2-6 Introduction to the Periodic Table Types of elements Trends in periods and groups Introduction of main group elements 2-7 The Concept of the Mole and the Avogadro Constant Definition of mole and Avogadro’s number Definition of molar mass 2-8 Using the Mole Concept in Calculations Converting between mass, number of moles, and number of atoms 4. 教学方法 多媒体教学: • Illustration of law of conservation of matter and law of multiple proportions • Illustration of key experiments leading to discoveries in atomic physics • Illustration of isotopes and mass spectra • Illustration of periodic table 互动教学: • How can cathod-ray experiment prove the existence of electrons • What is the identity of each of the radiation rays? • How can the gold-foil experiment tell us the structure of an atom?

There are 118 elements listed in the periodic table.Are there only 118 unique atoms in the world? Why is the relative atomic massnumbernever a whole number? 5. 教学评价 课后问题: Textbook,Chapter2:5,12,23.25,42,44,50,59,71 Chapter 3.Chemical Compounds 1.教学目标(五号宋体) Understand the properties of different chemicalcompounds and their formulas Know how to name common inorganic compounds 2. 教学重难点 教学重点: Properties of different chemical compoundsand their formulas The mole concept and chemical compounds Naming inorganic compounds 教学难点: Distinguish between molecularand ionic compounds Naming different types of inorganic compounds 3.教学内容 3-1 Types of Chemical Compounds and Their Formulas Molecular compounds Molecular formula and empirical formula Structure of molecules Ions and ionic compound 3-2 The Mole Concept and Chemical Compounds Relating molar mass,the Avogadro constant,and formula units of a compound Combining several factors in a calculation involving molar mass 3-3 Composition of Chemical Compounds Percent composition from formulas
• There are 118 elements listed in the periodic table. Are there only 118 unique atoms in the world? • Why is the relative atomic mass number never a whole number? 5. 教学评价 课后问题: • Textbook, Chapter 2: 5, 12, 23, 25, 42, 44, 50, 59, 71 Chapter 3. Chemical Compounds 1. 教学目标 (五号宋体) • Understand the properties of different chemical compounds and their formulas • Know how to name common inorganic compounds 2. 教学重难点 教学重点: • Properties of different chemical compounds and their formulas • The mole concept and chemical compounds • Naming inorganic compounds 教学难点: • Distinguish between molecular and ionic compounds • Naming different types of inorganic compounds 3. 教学内容 3-1 Types of Chemical Compounds and Their Formulas Molecular compounds Molecular formula and empirical formula Structure of molecules Ions and ionic compound 3-2 The Mole Concept and Chemical Compounds Relating molar mass, the Avogadro constant, and formula units of a compound Combining several factors in a calculation involving molar mass 3-3 Composition of Chemical Compounds Percent composition from formulas

Combustion analysis of C and H in compounds Empirical formulas from percent composition 3-5 Naming Compounds:Organic and Inorganic Compounds Definition of inorganic and organic compounds 3-6 Names and Formulas of Inorganic Compounds Naming molecular compounds Naming ionic compounds(binary ionic compounds:type I and type II;compounds containing polyatomic ions) Naming acids(Binary acids;oxyacids) Naming hydrates 3-7 Names and Formulas of Organic Compounds Root names for the parent hydrocarbon chains Root names for organic compounds of different classes 4.教学方法 多媒体教学: Illustration ofmolecular compounds and ionic compounds Illustration of predicting ions forming ● Illustration ofrules fornaming different types of inorganic compounds 互动教学 Do ionic compounds have molecular formulas? Can you see the patterns ofnames of polyatomic ions? How to name N2Os,LiCl,CuSO4,HCL,and HNO3? 5.教学评价 课后问题: Textbook,Chapter.3:11,12,16,19,37,43,57,58,61,62 Chapter 4.Chemical Reactions 1.教学目标(五号宋体) Know how to balance an equation Know different ways to present solution concentration
Combustion analysis of C and H in compounds Empirical formulas from percent composition 3-5 Naming Compounds: Organic and Inorganic Compounds Definition of inorganic and organic compounds 3-6 Names and Formulas of Inorganic Compounds Naming molecular compounds Naming ionic compounds (binary ionic compounds: type I and type II; compounds containing polyatomic ions) Naming acids (Binary acids; oxyacids) Naming hydrates 3-7 Names and Formulas of Organic Compounds Root names for the parent hydrocarbon chains Root names for organic compounds of different classes 4. 教学方法 多媒体教学: • Illustration of molecular compounds and ionic compounds • Illustration of predicting ions forming • Illustration of rules for naming different types of inorganic compounds 互动教学: • Do ionic compounds have molecular formulas? • Can you see the patterns of names of polyatomic ions? • How to name N2O5, LiCl, CuSO4,HCl, and HNO3? 5. 教学评价 课后问题: • Textbook, Chapter 3: 11, 12, 16, 19, 37, 43, 57, 58, 61, 62 Chapter 4. Chemical Reactions 1. 教学目标 (五号宋体) • Know how to balance an equation • Know different ways to present solution concentration

Understand the molar relationship in chemicalreactions Be able to solve stoichiometry related questions 2. 教学重难点 教学重点: Molar relationship in chemical reactions Solve different types of stoichiometry questions 教学难点: Additional conversion factors(volume,density,and percent composition)in reaction stoichiometry Solve limiting-reactant problems Theoretical,actual,and percent yield 3.教学内容 4-1 Chemical Reactions and Chemical Equations Definition of chemical reactions How to write chemical equations 4-2 Chemical Equations and Stoichiometry Molar relationship in chemical reactions Mass relationship in chemical reactions Additional conversion factors:volume,density,and percent composition 4-3 Chemical Reactions in Solution Solvent and solute Saturated solution and solubility Concentration of a solution(expressed as percent and molarity) 4-4 Determining the Limiting Reactant Identify the limiting reactant Solve limiting-reactant problems 4-5 Other Practical Matters in Reaction Stoichiometry Theoretical,actual,and percent yield Main reaction and side reactions Consecutive reactions,overall reaction,and simultaneous reactions
• Understand the molar relationship in chemical reactions • Be able to solve stoichiometry related questions 2. 教学重难点 教学重点: • Molar relationship in chemical reactions • Solve different types of stoichiometry questions 教学难点: • Additional conversion factors (volume, density, and percent composition) in reaction stoichiometry • Solve limiting-reactant problems • Theoretical, actual, and percent yield 3. 教学内容 4-1 Chemical Reactions and Chemical Equations Definition of chemical reactions How to write chemical equations 4-2 Chemical Equations and Stoichiometry Molar relationship in chemical reactions Mass relationship in chemical reactions Additional conversion factors: volume, density, and percent composition 4-3 Chemical Reactions in Solution Solvent and solute Saturated solution and solubility Concentration of a solution (expressed as percent and molarity) 4-4 Determining the Limiting Reactant Identify the limiting reactant Solve limiting-reactant problems 4-5 Other Practical Matters in Reaction Stoichiometry Theoretical, actual, and percent yield Main reaction and side reactions Consecutive reactions, overall reaction, and simultaneous reactions