
Basic Electronics Introductory Lecture Course for Technology and Instrumentation in Particle Physics 2011 Chicago,Illinois June9-14,2011 Presented By Gary Drake Argonne National Laboratory Session 3
Basic Electronics Introductory Lecture Course for Technology and Instrumentation in Particle Physics 2011 Chicago, Illinois June 9-14, 2011 Presented By Gary Drake Argonne National Laboratory Session 3

Session 3 Semiconductor Devices Basic Electronics-Special Lecture for TIPP 2011 2 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 2 Gary Drake, Argonne National Lab – Session 3 Session 3 Semiconductor Devices
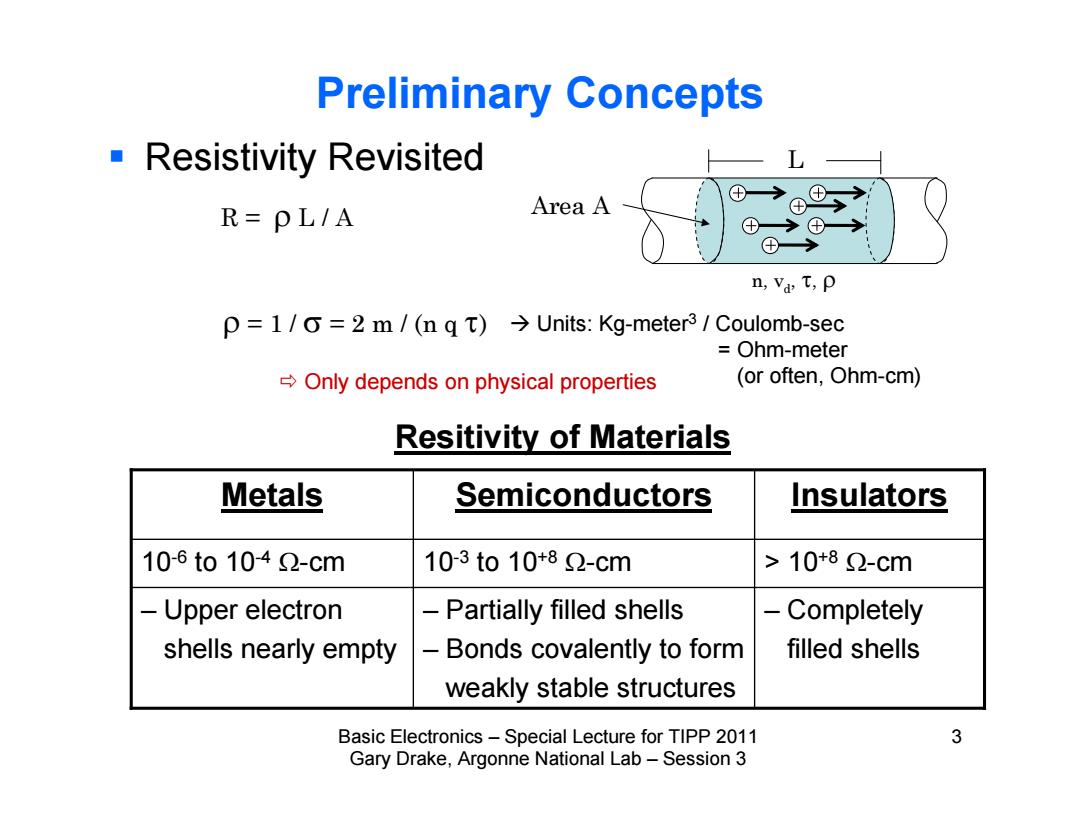
Preliminary Concepts Resistivity Revisited L Area A ①→ R=PL/A ⊕→① ⊕→ n,Va T,p p =1/0=2 m /(n q t)>Units:Kg-meter3 Coulomb-sec Ohm-meter Only depends on physical properties (or often,Ohm-cm) Resitivity of Materials Metals Semiconductors Insulators 10-6to1042-cm 103to10+82-cm >10*82-cm Upper electron -Partially filled shells - Completely shells nearly empty Bonds covalently to form filled shells weakly stable structures Basic Electronics-Special Lecture for TIPP 2011 3 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 3 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Resistivity Revisited = 1 / = 2 m / (n q ) R = L / A n, vd, , L Area A Units: Kg-meter3 / Coulomb-sec = Ohm-meter Only depends on physical properties (or often, Ohm-cm) – Completely filled shells – Partially filled shells – Bonds covalently to form weakly stable structures – Upper electron shells nearly empty > 10 10-6 to 10-4 -cm 10-3 to 10+8 -cm +8 -cm Metals Semiconductors Insulators Resitivity of Materials
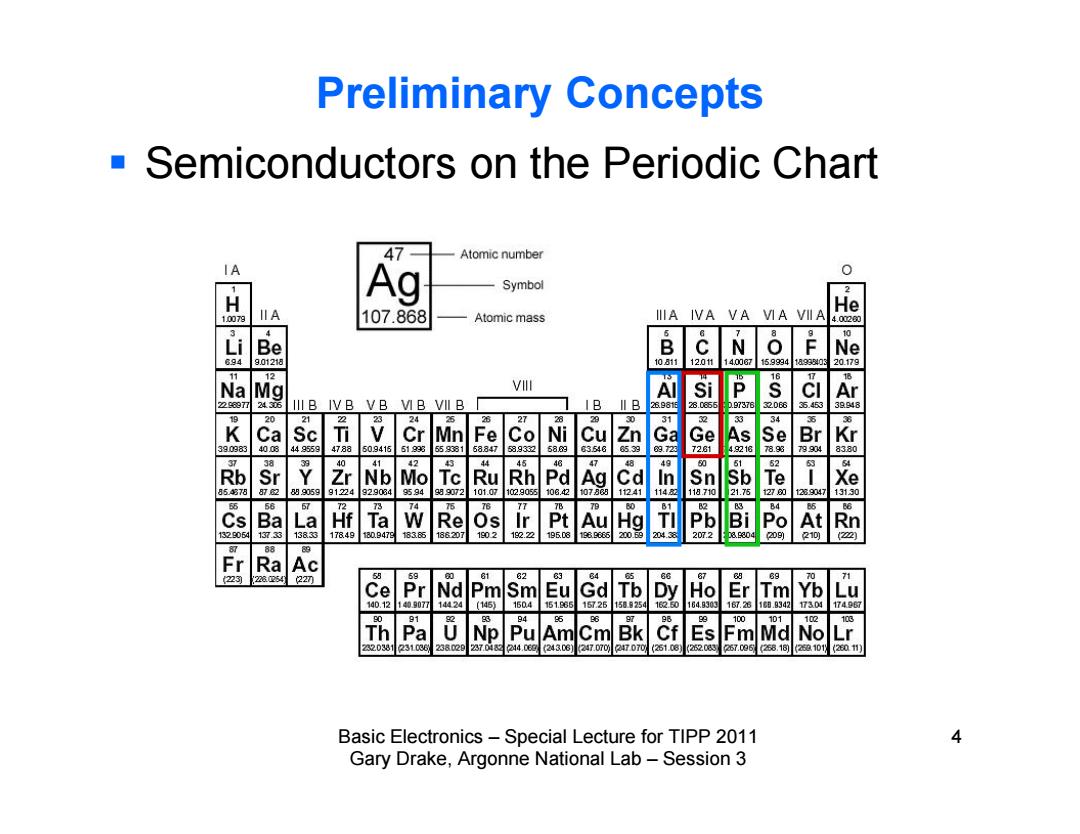
Preliminary Concepts Semiconductors on the Periodic Chart 47 Atomic number IA H Ag Symbol He 007 IIA 107.868 Atomic mass IIA IVA VA VIA VIIA 4.020 i Be N Ne 8g 901218 10811 12011 140087 15.99941ag38y0 20.179 2 Na Mg V Si P 22607T 24305 IlIB IVB VB VIB VII B B II B 898E 2B.0865 3 32D6 A 2 2 Ni 31 K Ca Sc Cr Mn Fe Co Cu Zn Br 390383 4008 449559 4788 509415 519g93 559381 58847 589332 68的 83548 539 Ge 990 Rb Sr r 4 5 Nb Mo Tc Ru Rh Pd Ag Cd 854378 8H 89905991224 g290349594 g99072 101.07 1029055106.2 t078 1241 1148 五 120 Cs B品 La Hf Ta W Re Os Pt Au Hg Pb Bi Po At Rn 132g06例 13733 13833 17849180.947E18385 188207 1g0212.22 19506 136g666 20059 204.38 207.2 0 100 88 Fr Ra Ac 2 228254 22刃 Pr Nd 31 2 Ce Pm Sm Eu Gd Tb Dy Ho Er Tm 140.12140.807714M24 (145) 1604 161.885 157261589254 162.50 164.9303 167.281C0.8342 17496 91 94 98 100 101 Th Pa ND Pu Am Cm Bk Es Fm 320381231036 238020237.0424.0e024306jl 247.D7027070261.06252.085 .6 2101 (2011】 Basic Electronics-Special Lecture for TIPP 2011 4 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 4 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Semiconductors on the Periodic Chart
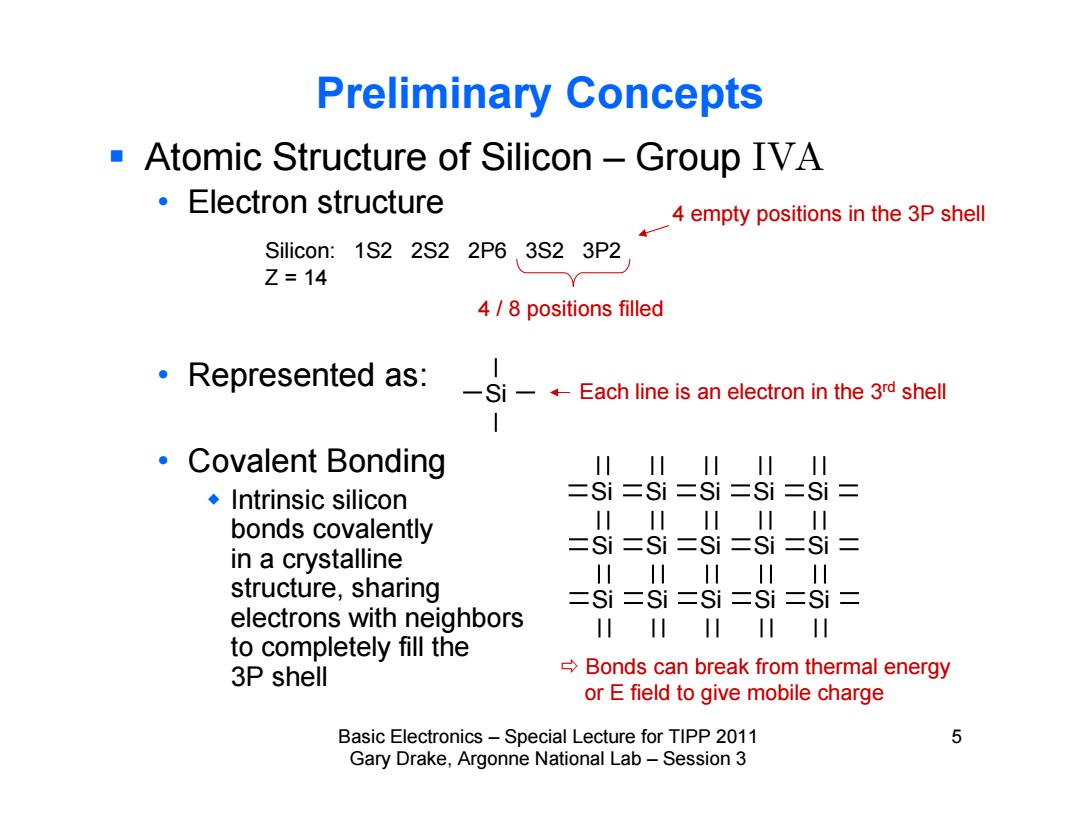
Preliminary Concepts Atomic Structure of Silicon-Group IVA ·Electron structure 4 empty positions in the 3P shell Silicon:1S2 2S2 2P6 3S2 3P2 Z=14 4/8 positions filled Represented as:SEach line is an electron in the 3d shell 1 ·Covalent Bonding I川川 川 I川 ◆Intrinsic silicon ESi =si =si =si si bonds covalently 川川川川川 =Si二Si二Si二Si二Si= in a crystalline 川川川川 川 structure,sharing 二Si=Si=Si二Si=Si= electrons with neighbors 川 川 to completely fill the 3P shell Bonds can break from thermal energy or E field to give mobile charge Basic Electronics-Special Lecture for TIPP 2011 5 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 5 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Atomic Structure of Silicon – Group IVA • Electron structure • Represented as: • Covalent Bonding Intrinsic silicon bonds covalently in a crystalline structure, sharing electrons with neighbors to completely fill the 3P shell Silicon: 1S2 2S2 2P6 3S2 3P2 Z = 14 Each line is an electron in the 3rd shell 4 / 8 positions filled Si Si Si Si Si Si Si Si Si Si Si Si Si Si Si Si 4 empty positions in the 3P shell Bonds can break from thermal energy or E field to give mobile charge
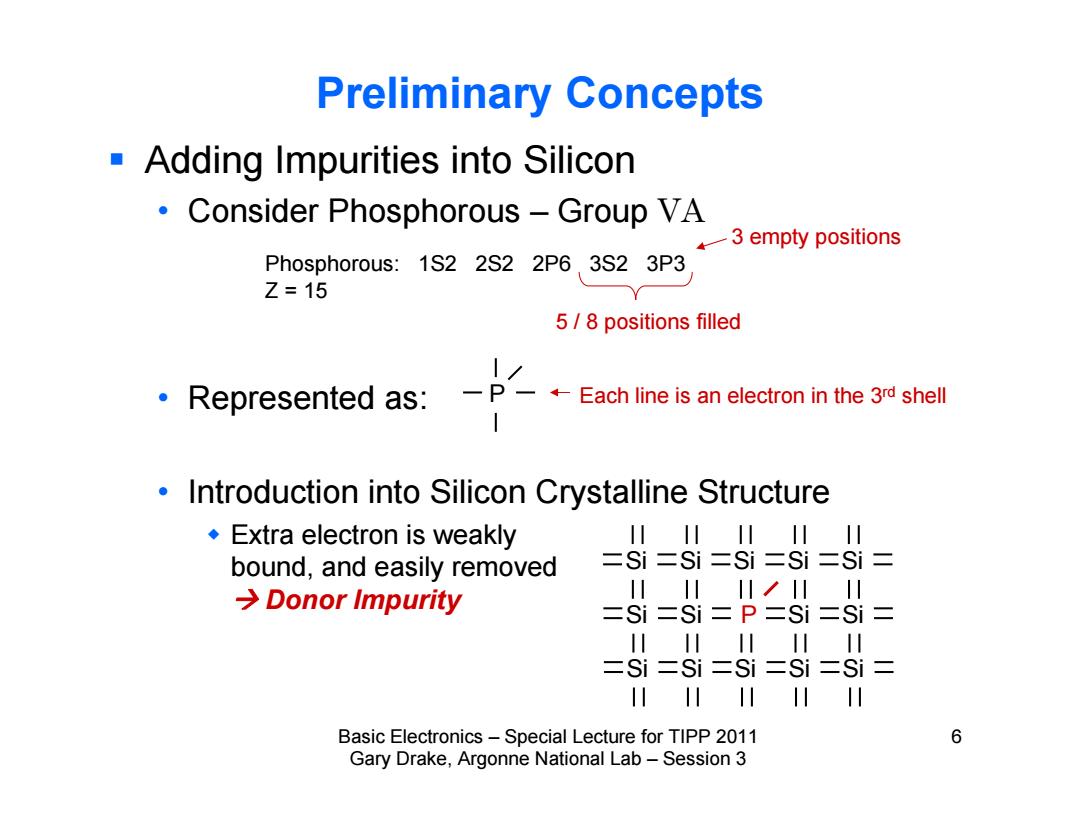
Preliminary Concepts Adding Impurities into Silicon Consider Phosphorous Group VA k一3 empty positions Phosphorous:1S2 2S2 2P6 3S2 3P3 Z=15 5/8 positions filled 1/ Represented as:-P-+Each line is an electron in the 3rd shell Introduction into Silicon Crystalline Structure Extra electron is weakly 川川川I川川 bound,and easily removed 二Si=Si二Si二Si二Si= →Donor Impurity 川川川/川IH =Si二Si=P二Si二Si= 川川川川 二Si二Si二Si二Si二Si= H川 Basic Electronics-Special Lecture for TIPP 2011 6 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 6 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Adding Impurities into Silicon • Consider Phosphorous – Group VA • Represented as: • Introduction into Silicon Crystalline Structure Extra electron is weakly bound, and easily removed Donor Impurity Phosphorous: 1S2 2S2 2P6 3S2 3P3 Z = 15 Each line is an electron in the 3rd shell 5 / 8 positions filled Si Si Si Si Si Si Si P Si Si Si Si Si Si Si P 3 empty positions
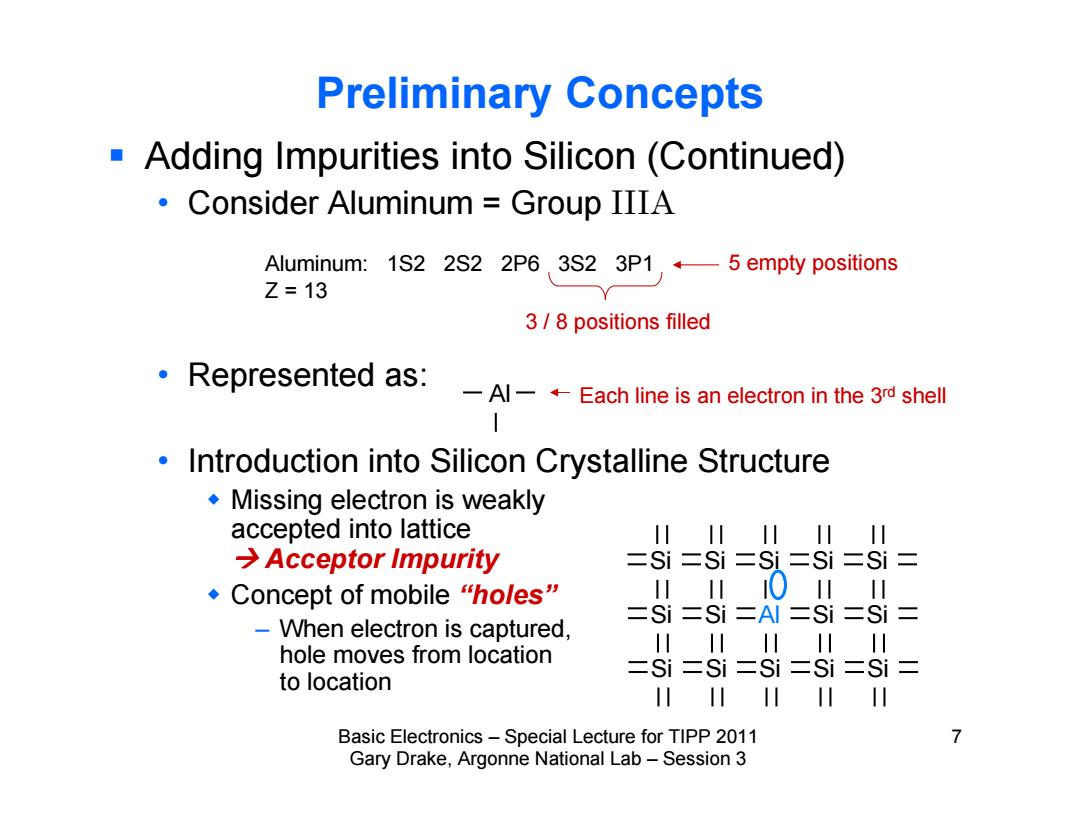
Preliminary Concepts Adding Impurities into Silicon(Continued) Consider Aluminum Group IIIA Aluminum:1S2 2S2 2P6 3S2 3P15 empty positions Z=13 3/8 positions filled Represented as:-Al-Each line is an electron in the 3 shell Introduction into Silicon Crystalline Structure Missing electron is weakly accepted into lattice 川 I川 川 I川 I川 →Acceptor Impurity 二Si二Si=Si=Si二Si= ◆Concept of mobile "holes” 0 川 =Si=Si=Al二Si=Si= -When electron is captured, hole moves from location 川川川川 川 二Si二Si二Si二Si=Si= to location I川 川 Basic Electronics-Special Lecture for TIPP 2011 7 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 7 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Adding Impurities into Silicon (Continued) • Consider Aluminum = Group IIIA • Represented as: • Introduction into Silicon Crystalline Structure Missing electron is weakly accepted into lattice Acceptor Impurity Concept of mobile “holes” – When electron is captured, hole moves from location to location Aluminum: 1S2 2S2 2P6 3S2 3P1 Z = 13 Each line is an electron in the 3rd shell 3 / 8 positions filled Si Si Si Si Si Si Si Al Si Si Si Si Si Si Si Al 5 empty positions
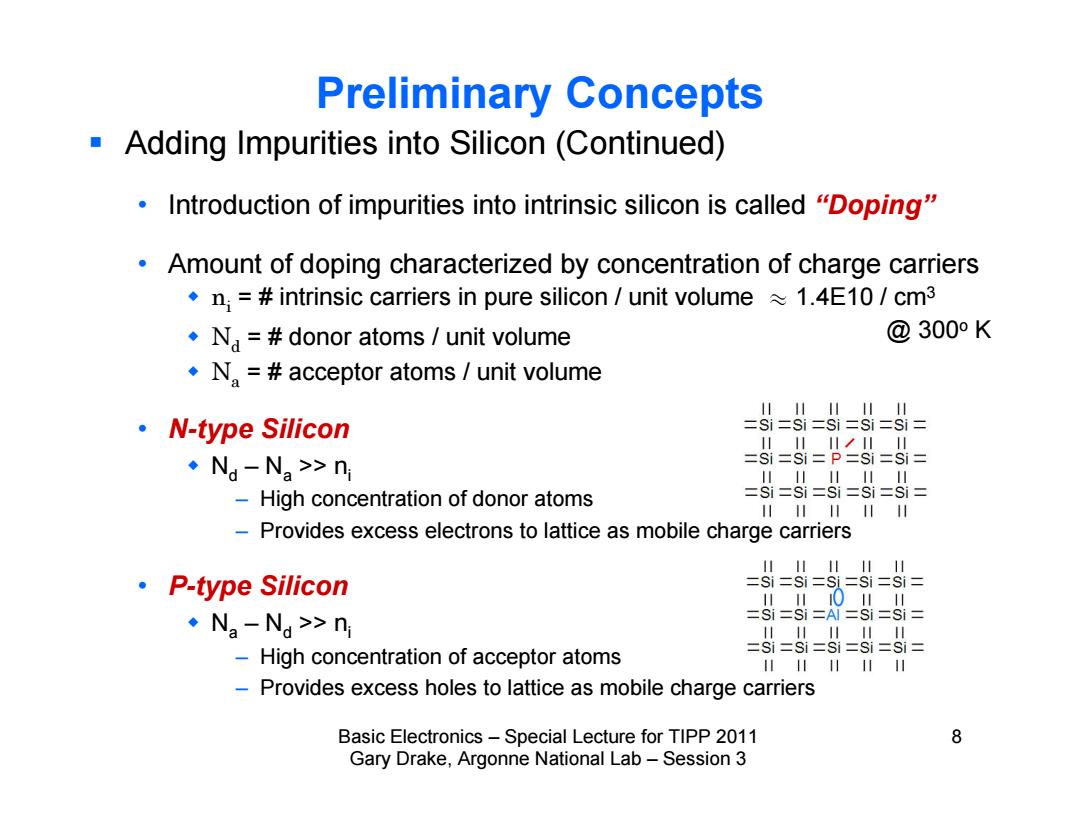
Preliminary Concepts Adding Impurities into Silicon (Continued) Introduction of impurities into intrinsic silicon is called "Doping" Amount of doping characterized by concentration of charge carriers .n;=#intrinsic carriers in pure silicon unit volume1.4E10/cm3 .N=#donor atoms unit volume @300°K .N.=acceptor atoms unit volume 1111川11 ·N-type Silicon =Si=si=si =si=si= 1川1◆川川 ·Na-Na>n si=si=P=Si=si= High concentration of donor atoms =S==8=60=6= 1111111 -Provides excess electrons to lattice as mobile charge carriers 1111川1 ·P-type Silicon =Si=si=si=si=si= 川1川0川日 ·Na-Na>n Si=Si =Al =Si=Si= 111川1 -High concentration of acceptor atoms =Si=si=si=si=si= 1111 -Provides excess holes to lattice as mobile charge carriers Basic Electronics-Special Lecture for TIPP 2011 8 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 8 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Adding Impurities into Silicon (Continued) • Introduction of impurities into intrinsic silicon is called “Doping” • Amount of doping characterized by concentration of charge carriers ni = # intrinsic carriers in pure silicon / unit volume y 1.4E10 / cm3 Nd = # donor atoms / unit volume Na = # acceptor atoms / unit volume • N-type Silicon Nd – Na >> ni – High concentration of donor atoms – Provides excess electrons to lattice as mobile charge carriers • P-type Silicon Na – Nd >> ni – High concentration of acceptor atoms – Provides excess holes to lattice as mobile charge carriers @ 300o K
Preliminary Concepts -Adding Impurities into Silicon(Continued) How to make use of mobile charge carriers Bonds can be broken by: Application of an Electric Field Basic principle of how integrated circuits work Application of Light>Photons impart energy >Basic principle of how photo cells work Use reverse principle for light emitting diodes(LEDs) -Heat→Kinetic Energy >Basic use for temperature sensors Generally a bad property for semiconductors... Basic Electronics-Special Lecture for TIPP 2011 9 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 9 Gary Drake, Argonne National Lab – Session 3 Preliminary Concepts Adding Impurities into Silicon (Continued) • How to make use of mobile charge carriers Bonds can be broken by: – Application of an Electric Field » Basic principle of how integrated circuits work – Application of Light Photons impart energy » Basic principle of how photo cells work » Use reverse principle for light emitting diodes (LEDs) – Heat Kinetic Energy » Basic use for temperature sensors » Generally a bad property for semiconductors…
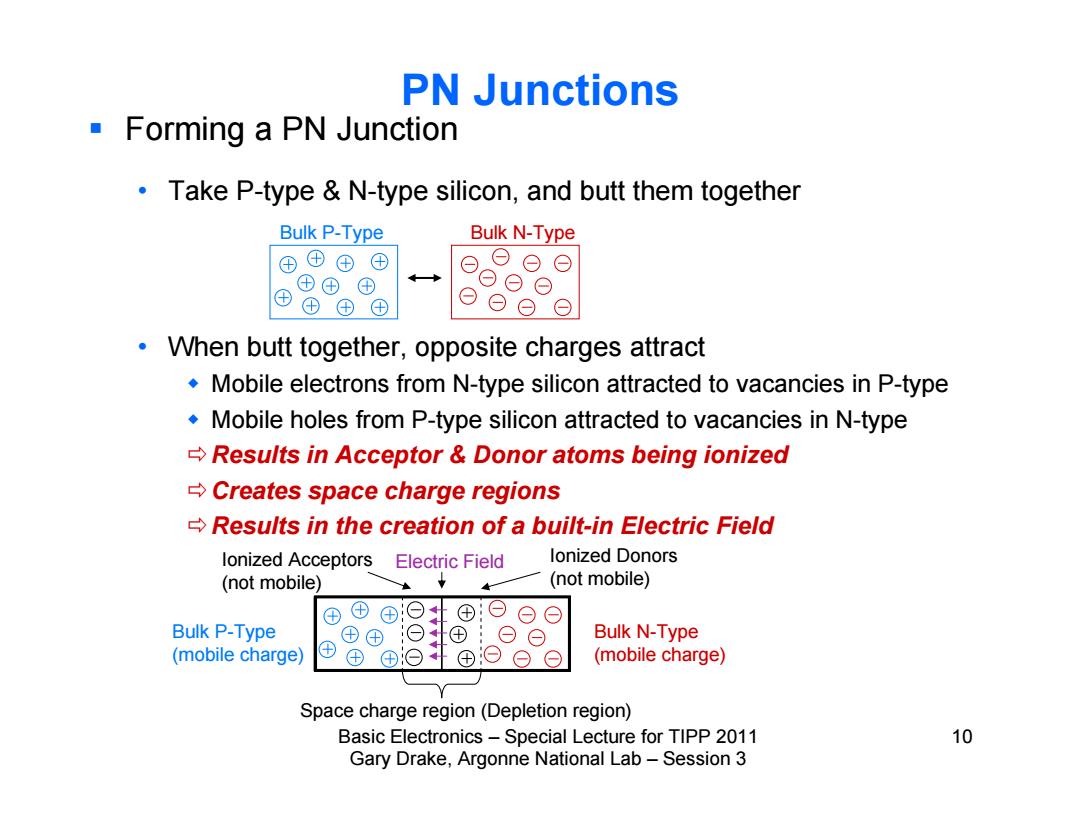
PN Junctions Forming a PN Junction Take P-type N-type silicon,and butt them together Bulk P-Type Bulk N-Type ⊕⊕© ⊕ ⊕⊕⊕ ⊕④田① When butt together,opposite charges attract Mobile electrons from N-type silicon attracted to vacancies in P-type Mobile holes from P-type silicon attracted to vacancies in N-type Results in Acceptor Donor atoms being ionized Creates space charge regions Results in the creation of a built-in Electric Field lonized Acceptors Electric Field lonized Donors (not mobile) (not mobile) ⊕ ©⊙ Bulk P-Type Bulk N-Type (mobile charge) ⊕ ⊕⊙ (mobile charge) Space charge region(Depletion region) Basic Electronics-Special Lecture for TIPP 2011 10 Gary Drake,Argonne National Lab-Session 3
Basic Electronics – Special Lecture for TIPP 2011 10 Gary Drake, Argonne National Lab – Session 3 PN Junctions Forming a PN Junction • Take P-type & N-type silicon, and butt them together • When butt together, opposite charges attract Mobile electrons from N-type silicon attracted to vacancies in P-type Mobile holes from P-type silicon attracted to vacancies in N-type Results in Acceptor & Donor atoms being ionized Creates space charge regions Results in the creation of a built-in Electric Field Bulk P-Type Bulk N-Type Bulk P-Type (mobile charge) Bulk N-Type (mobile charge) Ionized Acceptors (not mobile) Ionized Donors (not mobile) Electric Field Space charge region (Depletion region)