
Chapter 8 Atomic absorption spectrometry (AAS)
Chapter 8 Atomic absorption spectrometry (AAS)

●Basic principle of atomic absorption spectrometric analysis ● Structure of atomic absorption spectrophotometer ●Interference and elimination ●Quantitative analytical methods ●Sensitivity, characteristic concentration and detected limit ●brief introduction of atomic fluorescence spectrometry (AFS) 掌握原子吸收光谱分析法的基本原理,了解原子吸收光谱 仪的构造,重点掌握原子吸收分光光度定量分析方法。 Outlines
●Basic principle of atomic absorption spectrometric analysis ● Structure of atomic absorption spectrophotometer ●Interference and elimination ●Quantitative analytical methods ●Sensitivity, characteristic concentration and detected limit ●brief introduction of atomic fluorescence spectrometry (AFS) 掌握原子吸收光谱分析法的基本原理,了解原子吸收光谱 仪的构造,重点掌握原子吸收分光光度定量分析方法。 Outlines
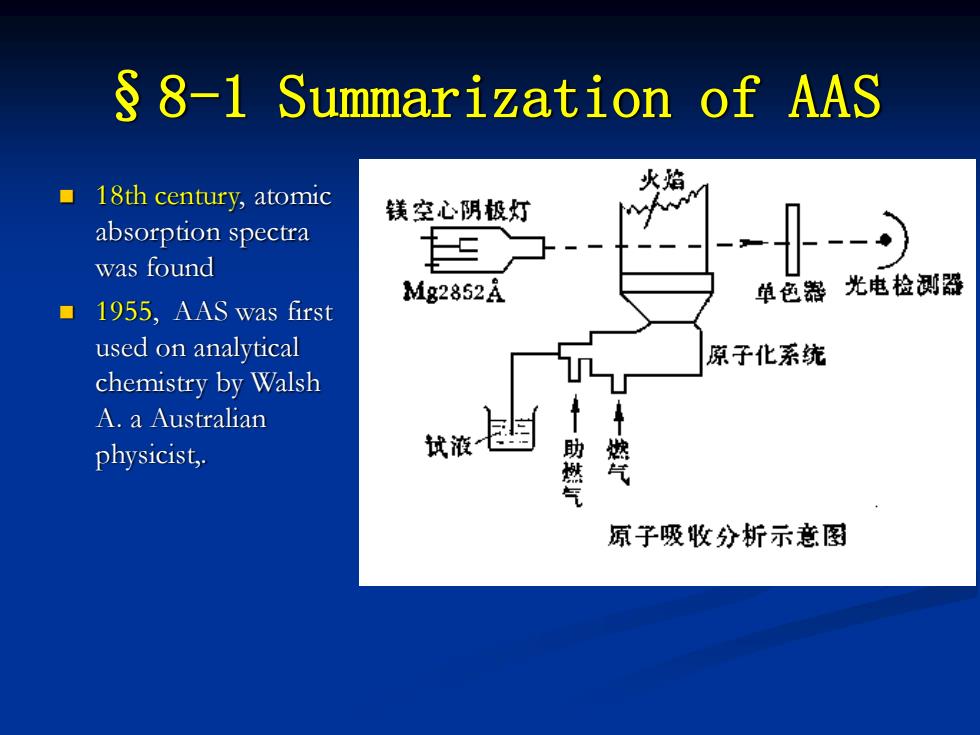
§8-1 Summarization of AAS ◼ 18th century, atomic absorption spectra was found ◼ 1955, AAS was first used on analytical chemistry by Walsh A. a Australian physicist
§8-1 Summarization of AAS ◼ 18th century, atomic absorption spectra was found ◼ 1955, AAS was first used on analytical chemistry by Walsh A. a Australian physicist
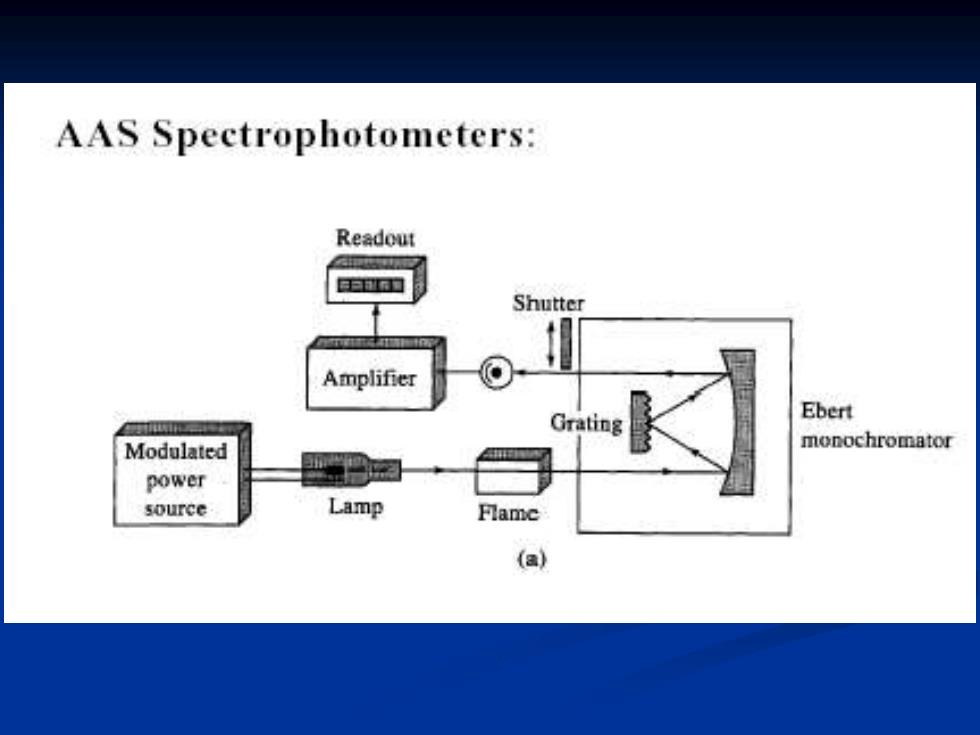
AAS Spectrophotometers: Readout E口 Shutter Amplifier Grating Ebert Modulated monochromator power source Lamp Flame (a)

原子吸收法的选择性高,干扰较少且易于克服。 由于原于的吸收线比发射线的数目少得多,这样谱线重 叠 的几率小得多。而且空心阴极灯一般并不发射那些 邻近波长的辐射线经,因此其它辐射线干扰较小。 原子吸收具有较高的灵敏度。 在原子吸收法的实验条件下,原子蒸气中基态 原于数 比激发态原子数多得多,所以测定的是大部分原 子。 原子吸收法 比发射法具有更佳的信噪比 这是由于激发态原子数的温度系数显著大于基态原子。 Comparison of AAS and AES 从仪器构造来看, 二者还有以下的异同点
原子吸收法的选择性高,干扰较少且易于克服。 由于原于的吸收线比发射线的数目少得多,这样谱线重 叠 的几率小得多。而且空心阴极灯一般并不发射那些 邻近波长的辐射线经,因此其它辐射线干扰较小。 原子吸收具有较高的灵敏度。 在原子吸收法的实验条件下,原子蒸气中基态 原于数 比激发态原子数多得多,所以测定的是大部分原 子。 原子吸收法 比发射法具有更佳的信噪比 这是由于激发态原子数的温度系数显著大于基态原子。 Comparison of AAS and AES 从仪器构造来看, 二者还有以下的异同点

● AAS intrinsically more sensitive than AES ● Similar atomization techniques to AES ● Addition of radiation source ● High temperature for atomization necessary flame and electrothermal atomization ● Very high temperature for excitation not necessary generally no plasma / arc / spark AAS Atomic absorption spectrometry
● AAS intrinsically more sensitive than AES ● Similar atomization techniques to AES ● Addition of radiation source ● High temperature for atomization necessary flame and electrothermal atomization ● Very high temperature for excitation not necessary generally no plasma / arc / spark AAS Atomic absorption spectrometry
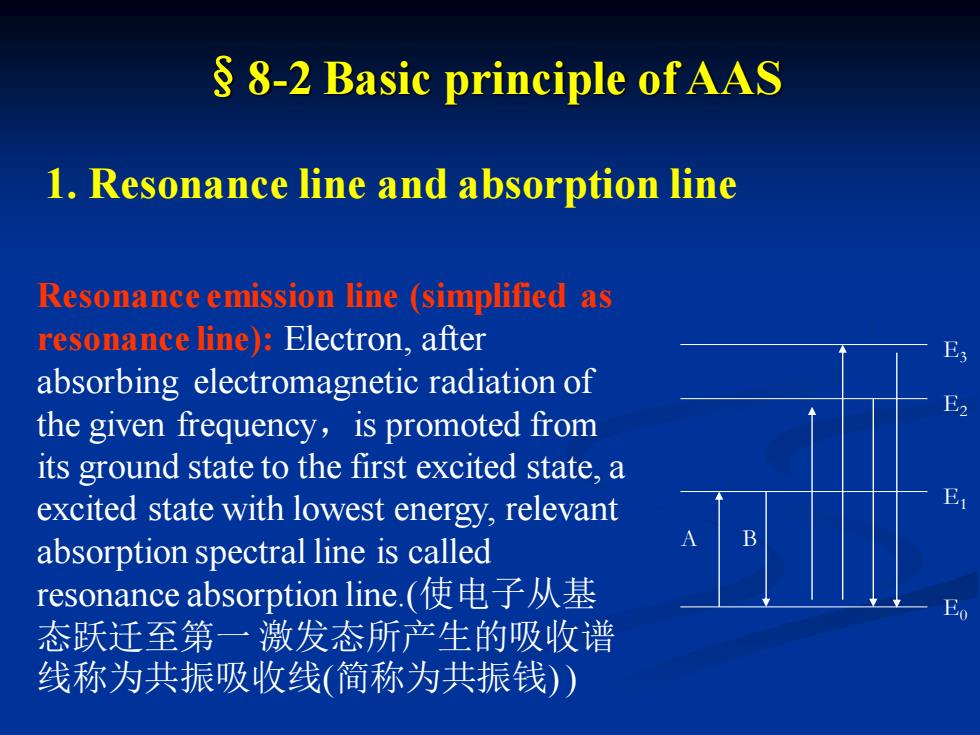
§8-2 Basic principle of AAS 1. Resonance line and absorption line E0 E1 E2 E3 A B Resonance emission line (simplified as resonance line): Electron, after absorbing electromagnetic radiation of the given frequency,is promoted from its ground state to the first excited state, a excited state with lowest energy, relevant absorption spectral line is called resonance absorption line.(使电子从基 态跃迁至第一 激发态所产生的吸收谱 线称为共振吸收线(简称为共振钱) )
§8-2 Basic principle of AAS 1. Resonance line and absorption line E0 E1 E2 E3 A B Resonance emission line (simplified as resonance line): Electron, after absorbing electromagnetic radiation of the given frequency,is promoted from its ground state to the first excited state, a excited state with lowest energy, relevant absorption spectral line is called resonance absorption line.(使电子从基 态跃迁至第一 激发态所产生的吸收谱 线称为共振吸收线(简称为共振钱) )

Resonance emission line (simplified as resonance line): The spectral line which electron emits when it directly returns (relax) form lowest electronic excited state to ground state is called resonance emission line which frequency equals that of electromagnetic radiation absorbed by electron when it is promoted from ground state to the first excited state. (电子从基 态跃迁到能量最低的激发态(称为第一激发态)时要吸收一定 频率的光,它再跃迁回基态时,则发射出同样频率的光(谱 线),这 种谱线称为共振发射线(简称共振线)。)
Resonance emission line (simplified as resonance line): The spectral line which electron emits when it directly returns (relax) form lowest electronic excited state to ground state is called resonance emission line which frequency equals that of electromagnetic radiation absorbed by electron when it is promoted from ground state to the first excited state. (电子从基 态跃迁到能量最低的激发态(称为第一激发态)时要吸收一定 频率的光,它再跃迁回基态时,则发射出同样频率的光(谱 线),这 种谱线称为共振发射线(简称共振线)。)

Characteristic spectral lines(特征谱线) Emphases For most atoms, resonance line is also sensitive line
Characteristic spectral lines(特征谱线) Emphases For most atoms, resonance line is also sensitive line

若将不同频率的光(强度为I0n ),) 通过原子蒸气(见右图),有一部 分光将被吸收.其透过光的强 度(即原子吸收共振线后光的强 度)与原子蒸气的宽度(即火焰的 宽度)的关系,同有色溶液吸收 光的情况完全类似,遵循朗伯 定律,即: Atomic vapor I0n In L Schematic diagram of atomic absorption Where Kn is absorption coefficient of atomic vapor to the electromagnetic with a frequency of n 2. Line profile and line broadening In = I0n e -KnL (eq.8-1)
若将不同频率的光(强度为I0n ),) 通过原子蒸气(见右图),有一部 分光将被吸收.其透过光的强 度(即原子吸收共振线后光的强 度)与原子蒸气的宽度(即火焰的 宽度)的关系,同有色溶液吸收 光的情况完全类似,遵循朗伯 定律,即: Atomic vapor I0n In L Schematic diagram of atomic absorption Where Kn is absorption coefficient of atomic vapor to the electromagnetic with a frequency of n 2. Line profile and line broadening In = I0n e -KnL (eq.8-1)