
CHAPTER 12 PHARMACEUTICALS AND THE ECONOMICS OF INNOVATION
CHAPTER 12 PHARMACEUTICALS AND THE ECONOMICS OF INNOVATION

Intro The pharmaceutical industry got its start in 1899, when Bayer,a German chemical company, introduced a painkiller called aspirin Today,the pharmaceutical industry is massive but tightly regulated This industry is an ideal setting to study both the economics of innovation and the economics of regulation. Bhattacharya,Hyde and Tu-HealthEconomics
Bhattacharya, Hyde and Tu – Health Economics Intro The pharmaceutical industry got its start in 1899, when Bayer, a German chemical company, introduced a painkiller called aspirin Today, the pharmaceutical industry is massive but tightly regulated This industry is an ideal setting to study both the economics of innovation and the economics of regulation

THE LIFE CYCLE OF A DRUG Ch 12 I Pharmaceuticals and the economics of innovation
Ch 12 | Pharmaceuticals and the economics of innovation THE LIFE CYCLE OF A DRUG
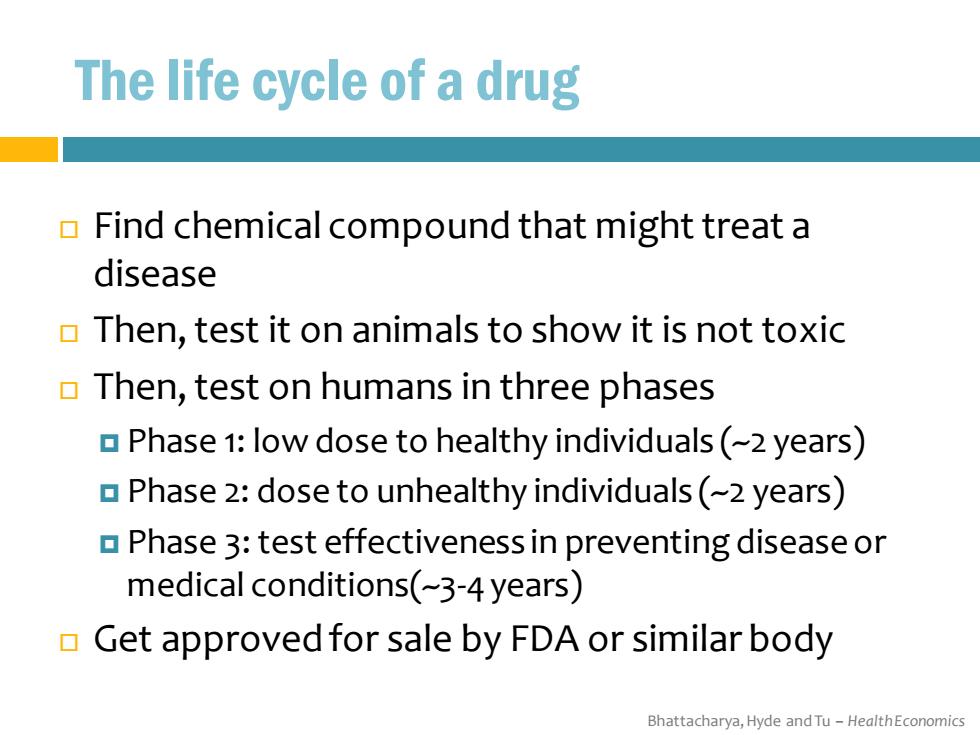
The life cycle of a drug Find chemical compound that might treat a disease Then,test it on animals to show it is not toxic Then,test on humans in three phases Phase 1:low dose to healthy individuals(-2 years) Phase 2:dose to unhealthy individuals(-2 years) Phase 3:test effectiveness in preventing disease or medical conditions(~-3-4 years) Get approved for sale by FDA or similar body Bhattacharya,Hyde and Tu-HealthEconomics
Bhattacharya, Hyde and Tu – Health Economics The life cycle of a drug Find chemical compound that might treat a disease Then, test it on animals to show it is not toxic Then, test on humans in three phases Phase 1: low dose to healthy individuals (~2 years) Phase 2: dose to unhealthy individuals (~2 years) Phase 3: test effectiveness in preventing disease or medical conditions(~3-4 years) Get approved for sale by FDA or similar body

The life cycle of a drug Once the drug is approved for sale,the drug company has a temporary legal monopoly protected by a patent(17 years in the US) This is the company's chance to recoup the millions of dollars spent on testing After that time is up,other companies can produce the same drug cheaply and profits decrease sharply Bhattacharya,Hyde and Tu-HealthEconomics
Bhattacharya, Hyde and Tu – Health Economics The life cycle of a drug Once the drug is approved for sale, the drug company has a temporary legal monopoly protected by a patent (17 years in the US) This is the company’s chance to recoup the millions of dollars spent on testing After that time is up, other companies can produce the same drug cheaply and profits decrease sharply

DRUG DEVELOPMENT Ch 12 Pharmaceuticals and the economics of innovation
Ch 12 | Pharmaceuticals and the economics of innovation DRUG DEVELOPMENT

Drug development is costly Hard to find a promising chemical in the first place Only 21.5%of drugs that enter Phase I pass to Phase Ill The whole process can cost $500 million or more to bring a drug to the point of approval Bhattacharya,Hyde and Tu-HealthEconomics
Bhattacharya, Hyde and Tu – Health Economics Drug development is costly Hard to find a promising chemical in the first place Only 21.5% of drugs that enter Phase I pass to Phase III The whole process can cost $500 million or more to bring a drug to the point of approval

PATENTS Ch 12 Pharmaceuticals and the economics of innovation
Ch 12 | Pharmaceuticals and the economics of innovation PATENTS

How do we induce companies to make these costly investments? Patents create a legal monopoly and hence the opportunity for monopoly profits In practice,only the top 30%of drugs pay for themselves Bhattacharya,Hyde and Tu-HealthEconomics
Bhattacharya, Hyde and Tu – Health Economics How do we induce companies to make these costly investments? Patents create a legal monopoly and hence the opportunity for monopoly profits In practice, only the top 30% of drugs pay for themselves

How strong should patents be? Patent strength Downside of stronger patents Customers have to pay monopoly prices for a longer period Less incentive for further innovation by same company Legal barriers to subsequent innovation by another company But if patents are too weak,no incentive to develop new drugs! Bhattacharya,Hyde and Tu-HealthEconomics
Bhattacharya, Hyde and Tu – Health Economics How strong should patents be? Downside of stronger patents Customers have to pay monopoly prices for a longer period Less incentive for further innovation by same company Legal barriers to subsequent innovation by another company But if patents are too weak, no incentive to develop new drugs!