
Atomic Physics RESERVE BANKOP NEW ZEALAND 00g AD273409 RITHERFORD NELSON THIS NOTE JS LEGAL TENDER EOR ONE HUNDRED DOLLARS
Atomic Physics

Agenda today 1.Spectrum of hydrogen atoms 2.Bohr's model for hydrogen atom 3.Schodiger's equation for hydrogen atom 4.The wave function of hydrogen atom
Agenda today 1. Spectrum of hydrogen atoms 2. Bohr’s model for hydrogen atom 3. Schodiger’s equation for hydrogen atom 4. The wave function of hydrogen atom
Before atom 肝 生 Hot Met 腎 Earth 肺 脾
Before atom
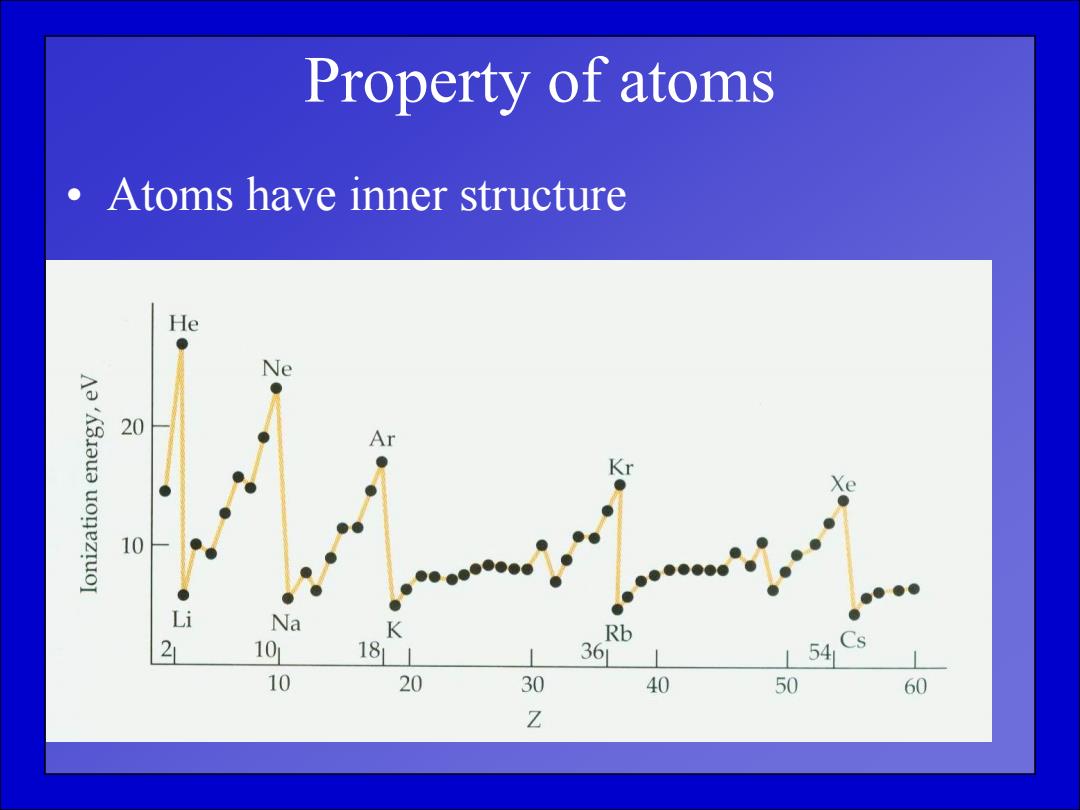
Property of atoms Atoms have inner structure He Ne Aa'A3aua uonezruoI 20 Ar 10 ● Li Na K Rb 21 101 18 361 L54 10 20 30 40 50 60 Z
Property of atoms • Atoms have inner structure
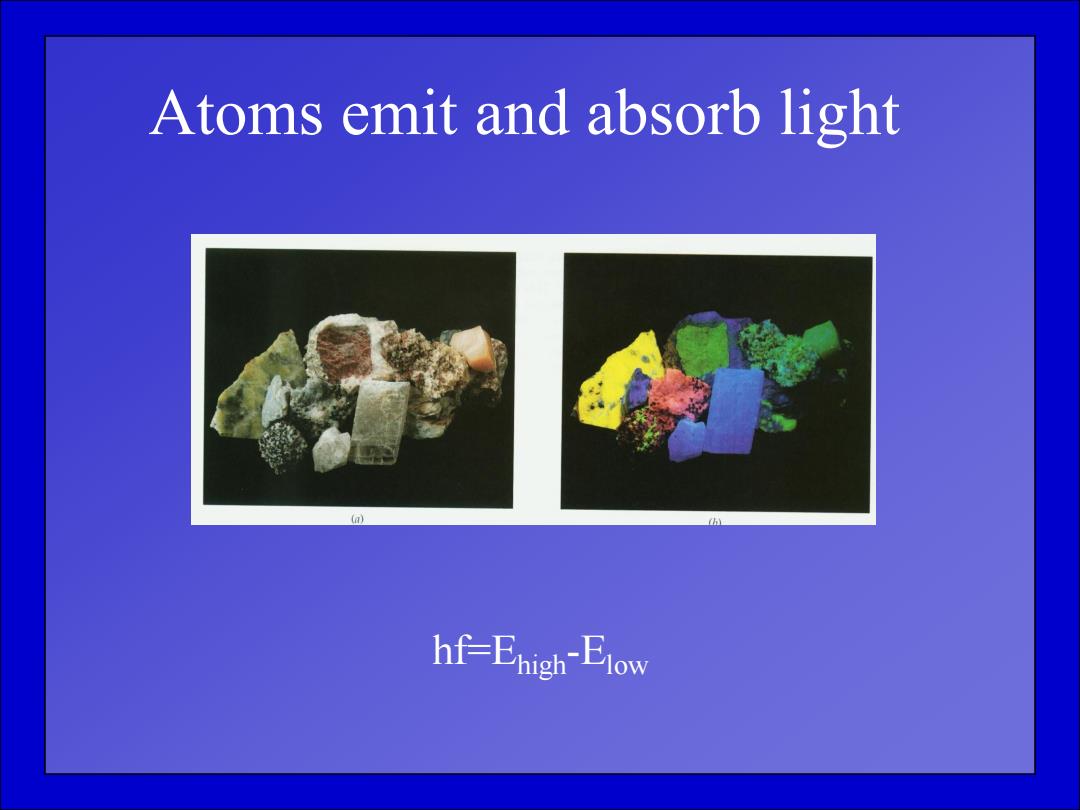
Atoms emit and absorb light hf-Enigh-Elow
Atoms emit and absorb light hf=Ehigh-Elow

Atoms have angular momentum and magnetism
Atoms have angular momentum and magnetism
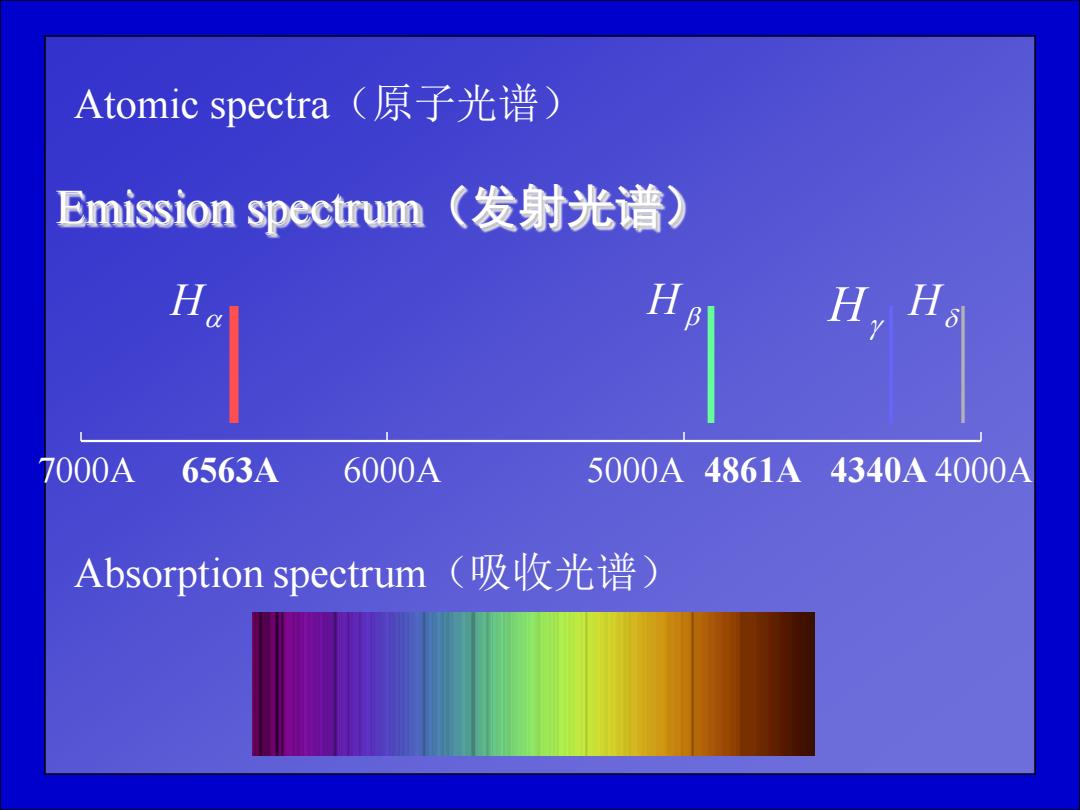
Atomic spectra(原子光谱) Emission spectrum(发射光谱) H,H。 7000A 6563A 6000A 5000A4861A4340A4000A Absorption spectrum(吸收光谱)
Atomic spectra(原子光谱) Emission spectrum(发射光谱) 7000A 6563A 6000A 5000A 4861A 4340A 4000A H H H H Absorption spectrum(吸收光谱)
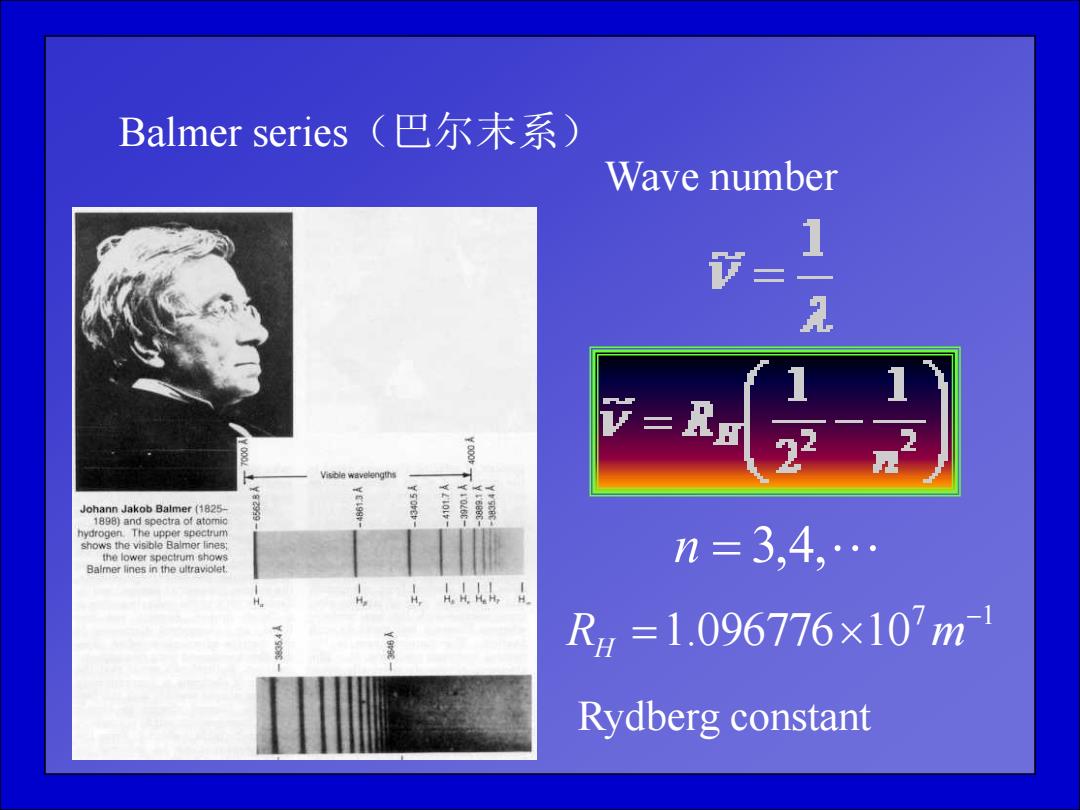
Balmer series(巴尔末系) Wave number 1 节= 2 v=R Johann Jakob Balmer (1825- 1898)and spectra of atomic the lower spectrum shows n=3,4, Baimer lines in the ultraviolet Rg=1.096776×10m Rydberg constant
Balmer series(巴尔末系) n 3,4, Wave number 7 1 1.096776 10 RH m Rydberg constant

Lyman series -非 n=2,3, Paschen series n=4,5, Brackett series V-Ru n=5,6,…
Lyman series n 2,3, Paschen series n 4,5, Brackett series n 5,6,
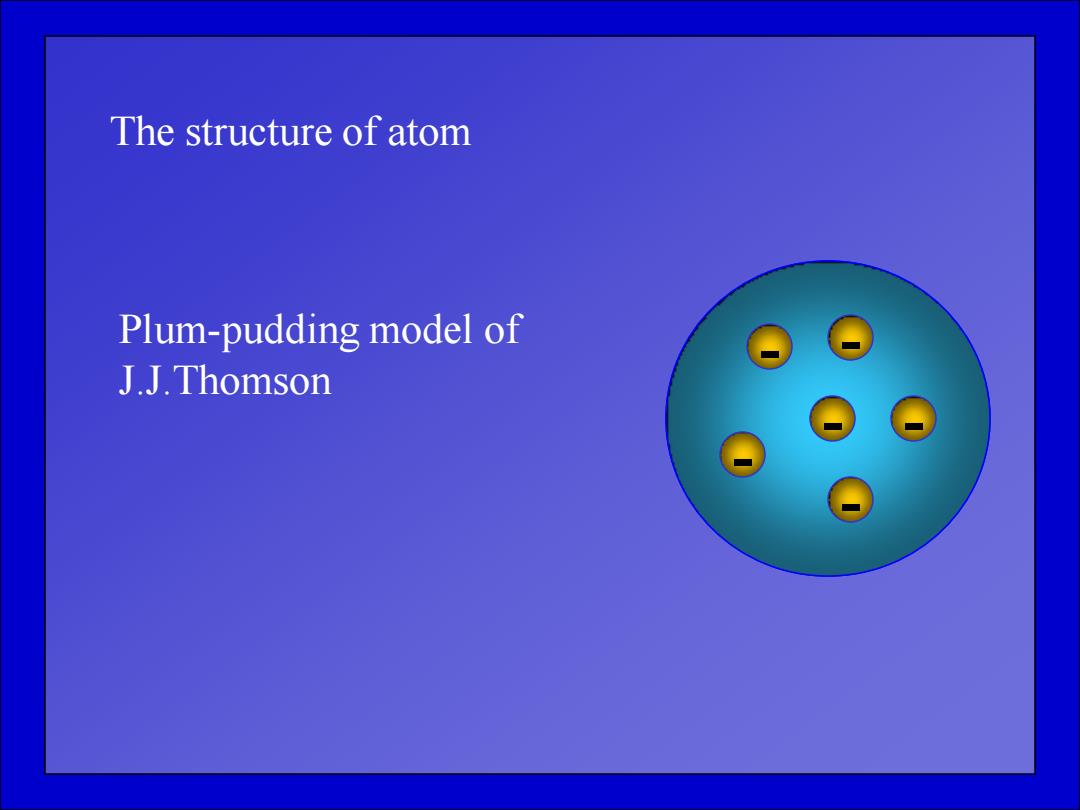
The structure of atom Plum-pudding model of J.J.Thomson
The structure of atom - - - - - - Plum-pudding model of J.J.Thomson