
Multi-electron atom Chemical bond
Multi-electron atom Chemical bond

Agenda today 1.Spin of particle 2.the exclusion principle 3.X-ray spectrum 4.The nature of chemical bond
Agenda today 1. Spin of particle 2. the exclusion principle 3. X-ray spectrum 4. The nature of chemical bond

Spin of electron: Force on magnetic dipole in a non-uniform magnetic field ve 4=IS= 元r2 (B 2nr e e mvr= 2m 2m F2=H: @B. 02
Spin of electron: Force on magnetic dipole in a non-uniform magnetic field -e
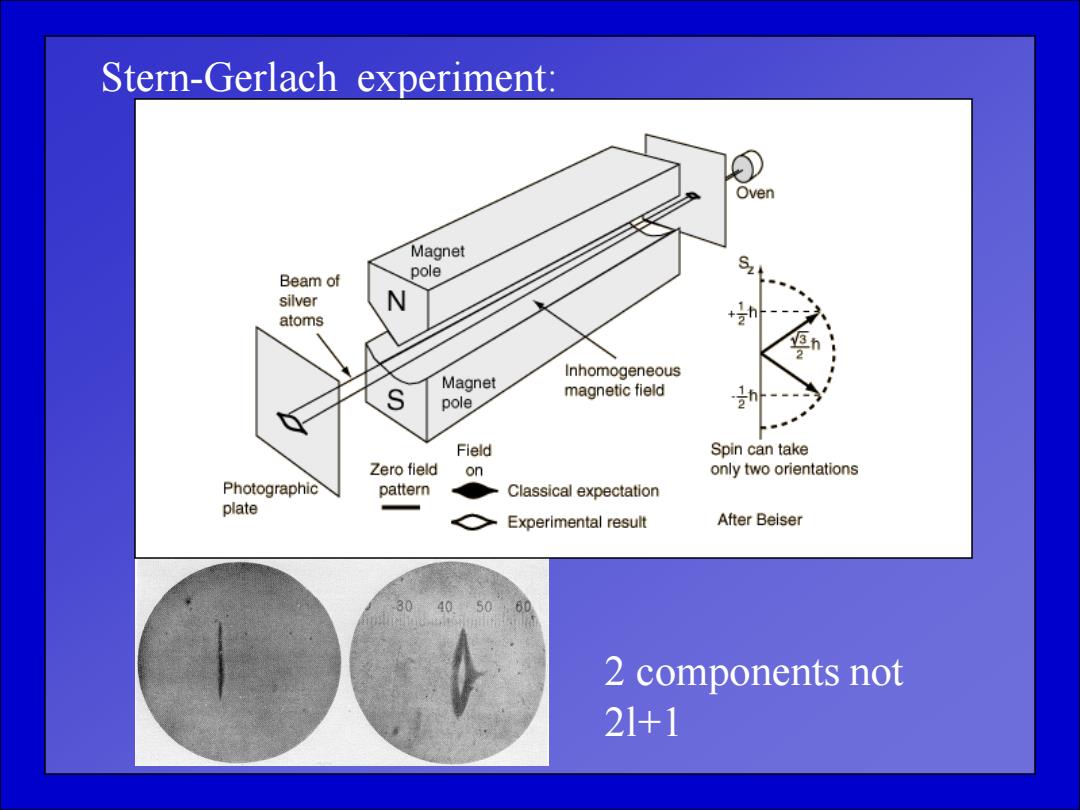
Stern-Gerlach experiment: Oven Magnet Beam of pole silver N atoms Magnet Inhomogeneous S magnetic field pole Field Spin can take Zero field on only two orientations Photographic pattern Classical expectation plate Experimental result After Beiser 2 components not 21+1
Stern-Gerlach experiment: 2 components not 2l+1
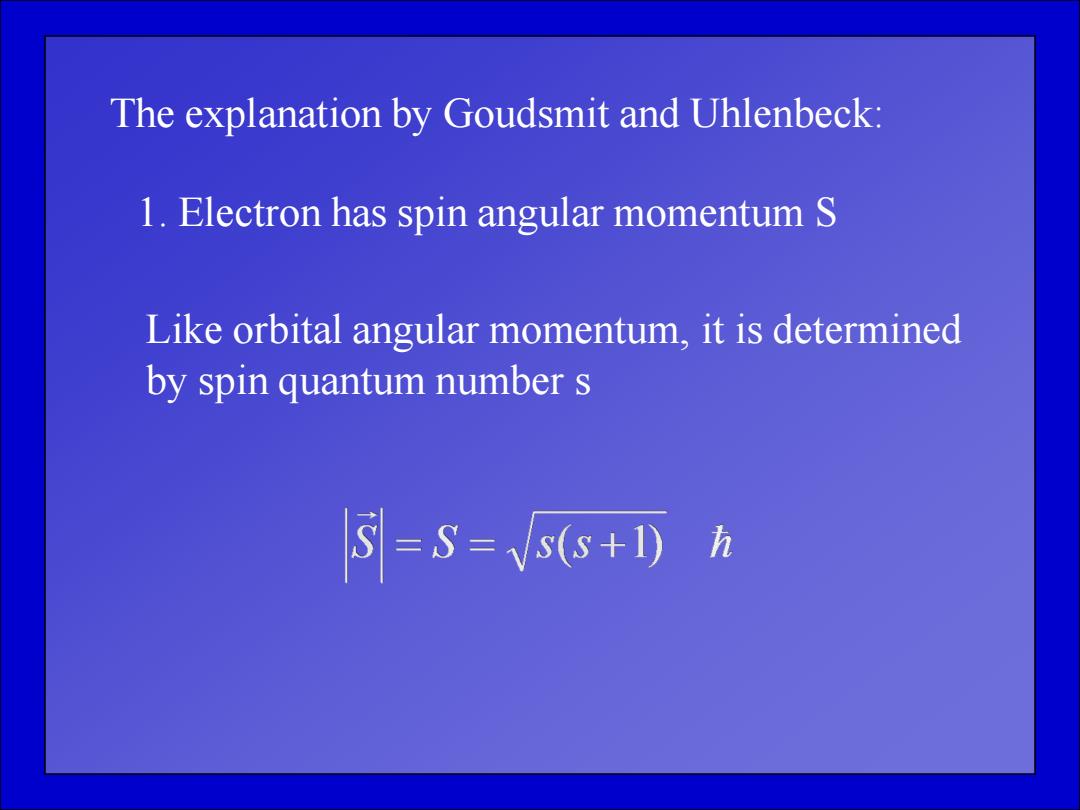
The explanation by Goudsmit and Uhlenbeck: 1.Electron has spin angular momentum S Like orbital angular momentum,it is determined by spin quantum number s =S=5s+D方
The explanation by Goudsmit and Uhlenbeck: 1. Electron has spin angular momentum S Like orbital angular momentum, it is determined by spin quantum number s

Sie beide sind jung.Sie konnen sich eine Dummheit leisten
‘Sie beide sind jung. Sie kÖnnen sich eine Dummheit leisten

2.Spin angular momentum is also space quantized 2s+1=2,s=1/2 Spin magnetic quantum number m Like orbital magnetic quantum number m2=-l,-l+1,…,l-1,l m,=-s,+s=-1/2,+1/2 S,=m,=-2’+2
2s+1=2 , s=1/2 2. Spin angular momentum is also space quantized. Spin magnetic quantum number ms Like orbital magnetic quantum number ms= -s ,+ s = -1/2,+1/2
A110 the application:NMR p110
the application: NMR
Mystical Baseline L ACCL GOM Lhsu同L Caudate 65 R GTM R SPL R LPI 65432

Fermion and Boson(费米子和玻色子) Particles with half-integer spins are called fermions Electron proton,neutron are fermions Particle with integer spins are called bosons Photon
Fermion and Boson (费米子和玻色子) Particles with half-integer spins are called fermions Electron , proton, neutron are fermions Particle with integer spins are called bosons Photon