
光化学转换材料 一.太阳能光解水制氢材料 二.光催化C02转换材料 三.光电催化转换材料 上泽充通大粤 SHANGIAI JIAO TONG UNIVERSTTY
光化学转换材料 一.太阳能光解水制氢材料 二.光催化CO2转换材料 三.光电催化转换材料
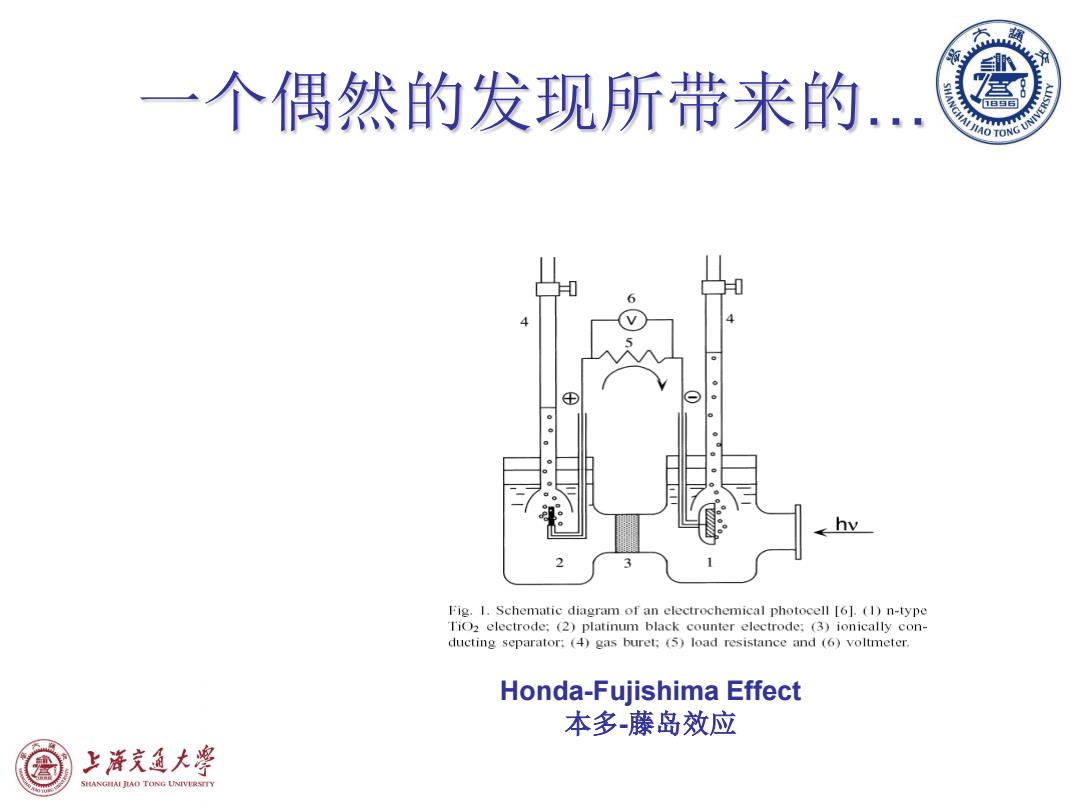
一个偶然的发现所带来的. Fig.1.Schematic diagram of an electrochemical photocell [6].(1)n-type TiO2 electrode:(2)platinum black counter electrode:(3)ionically con- ducting separator:(4)gas buret;(5)load resistance and (6)voltmeter. Honda-Fujishima Effect 本多-藤岛效应 上泽充通大粤 SHANGIAI JIAO TONG UNIVERSTTY
一个偶然的发现所带来的… Honda-Fujishima Effect 本多-藤岛效应
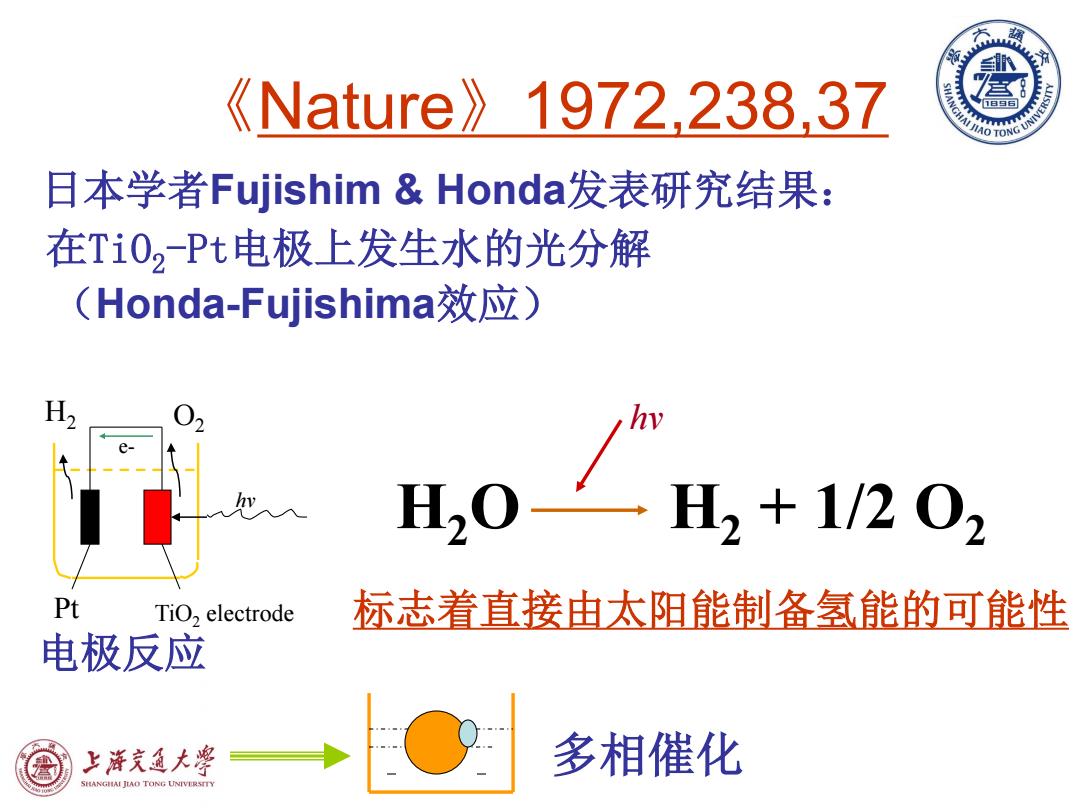
《Nature》1972,238,37 日本学者Fujishim&Honda发表研究结果: 在Ti02Pt电极上发生水的光分解 (Honda-Fujishima效应) H, hi HQ H2+1202 Pt TiO,electrode 标志着直接由太阳能制备氢能的可能性 电极反应 上游充通大粤 多相催化 SHANGIAI JIAO TONG UNIVERSTTY
《Nature》1972,238,37 日本学者Fujishim & Honda发表研究结果: 在TiO2-Pt电极上发生水的光分解 (Honda-Fujishima效应) TiO2 electrode 标志着直接由太阳能制备氢能的可能性 e- hv H2 O2 Pt H2O H2 + 1/2 O2 hv 多相催化 电极反应
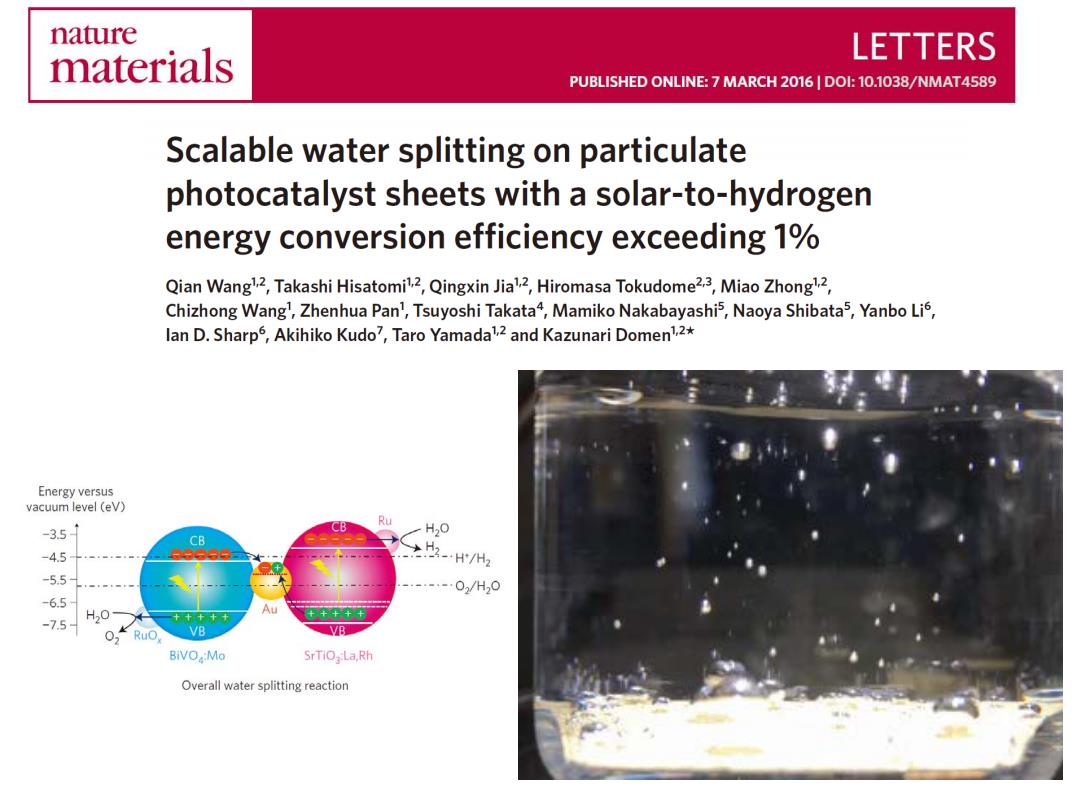
nature materials LETTERS PUBLISHED ONLINE:7 MARCH 2016 DOI:10.1038/NMAT4589 Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1% Qian Wang2,Takashi Hisatomi2,Qingxin Jia2,Hiromasa Tokudome2.3,Miao Zhong2, Chizhong Wang',Zhenhua Pan,Tsuyoshi Takata4,Mamiko Nakabayashis,Naoya Shibata5,Yanbo Lis, lan D.Sharp5,Akihiko Kudo7,Taro Yamada2 and Kazunari Domen12* Energy versus vacuum level(eV) 351 CB Ru H20 CB -4.5 ≥H/州2 -5.5 02/H20 -6.5 H20 AU -7.5 0 Ru, VB VB BiVO :Mo SrTiO,:La,Rh Overall water splitting reaction

In 1874,Jules Verne said: HIAO TONG "Yes,my friends,I believe that water will one day be employed as fuel, that hydrogen and oxygen which constitute it,used singly or together, will furnish an inexhaustible source of heat and light,of an intensity of which coal is not capable....water will be the coal of the future". From 《Mysterious Island》 140年前的幻想-凡尔纳的科幻小说《神秘岛》: “我相信冠会有一天可以用水来做燃料,组沫水的氦和氧可以 单浊或合在一起被使用。这将为热和光提供无狐的来源,所供 给的光和热是煤炭所无法达對的。所以我相信,一且媒矿祛竭 ”我们?会用水来供热和取暖。水将是未来的煤炭。 上泽充通大粤 SHANGIAI JIAO TONG UNIVERSTTY
“我相信总会有一天可以用水来做燃料,组成水的氢和氧可以 单独或合在一起被使用。这将为热和光提供无限的来源,所供 给的光和热是煤炭所无法达到的。所以我相信,一旦煤矿枯竭 了,我们将会用水来供热和取暖。水将是未来的煤炭。” 140年前的幻想------凡尔纳的科幻小说《神秘岛》: "Yes, my friends, I believe that water will one day be employed as fuel, that hydrogen and oxygen which constitute it, used singly or together, will furnish an inexhaustible source of heat and light, of an intensity of which coal is not capable….water will be the coal of the future". In 1874, Jules Verne said: From《Mysterious Island》

Hydrogen Movement (1974-2004) 15WHEC,Yokohama,Japan,2004 International Conferences 14WHEC,Montreal,Canada,2002 13WHEC,Beijing,2000 12WHEC,Buenos Aires,1998 11WHEC,Stuttgart,1996 10WHEC,Cocoa Beach,1994 9WHEC,Paris,1992 8WHEC,Honolulu,1990 7WHEC,Moscow,1988 6WHEC,Vienna,1986 5WHEC,Toronto,1984 4WHEC,Pasadena,1982 3WHEC,Tokyo,1980 2WHEC,Zurich,1978 1WHEC,Miami Beach,1976 THEME Conference,1974 1974 2004 上泽究通大皇 SHANGHAI JLAO TONG UNIVERSTTY
15WHEC, Yokohama, Japan, 2004 14WHEC, Montreal, Canada , 2002 13WHEC, Beijing, 2000 12WHEC, Buenos Aires, 1998 11WHEC, Stuttgart, 1996 10WHEC, Cocoa Beach, 1994 9WHEC, Paris, 1992 8WHEC, Honolulu, 1990 7WHEC, Moscow, 1988 6WHEC, Vienna, 1986 5WHEC, Toronto, 1984 4WHEC, Pasadena, 1982 3WHEC, Tokyo, 1980 2WHEC, Zurich, 1978 1WHEC, Miami Beach, 1976 THEME Conference, 1974 Hydrogen Movement (1974-2004) International Conferences 1974 2004
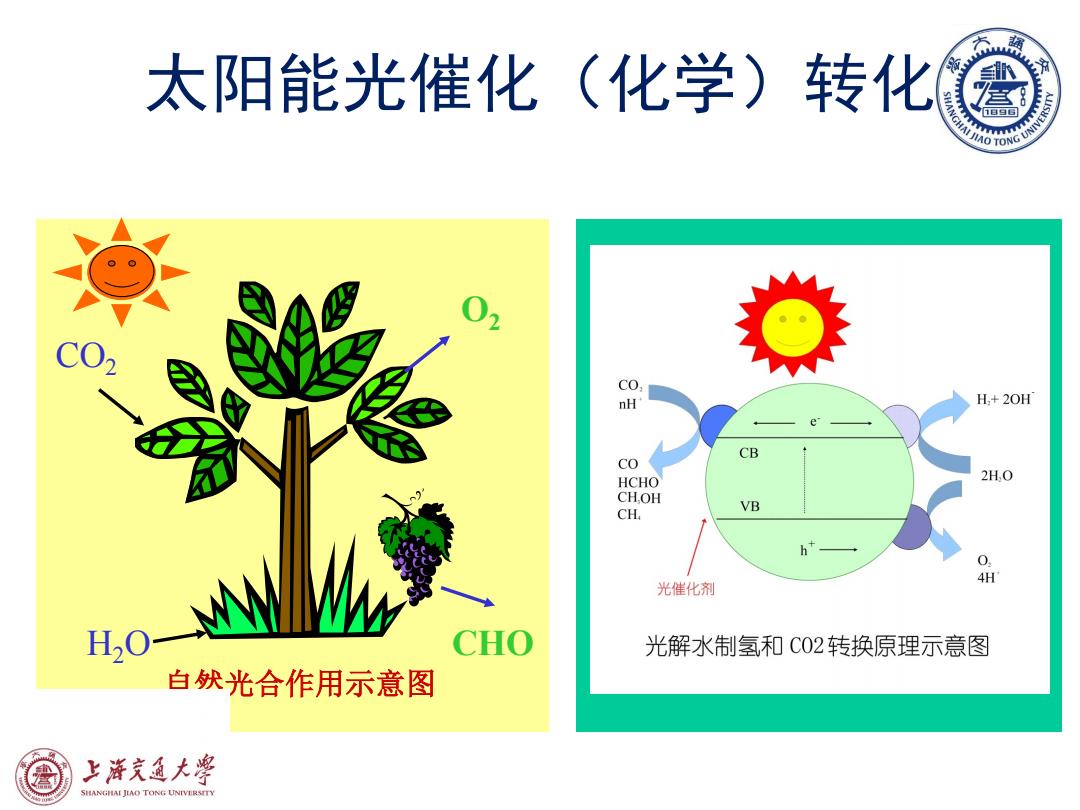
太阳能光催化(化学)转化 02 CO nH H,+20H CB CO HCHO 2H,O CHOH VB CH 0 4H O-W 光催化剂 CHO 光解水制氢和C02转换原理示意图 白然光合作用示意图 上泽究通大皇 SHANGHAI JLAO TONG UNIVERSTTY
太阳能光催化(化学)转化 CO2 H2O CHO O2 自然光合作用示意图

一、太阳能光解水制氢材料 Photocatalytic materials for solar hydrogen from water splitting 。1.光催化制氢基本原理 ·2.光催化制氢反应体系 ·3.光催化制氢材料 上游充通大粤 SHANGHAI JLAO TONG UNIVERSTTY
一、太阳能光解水制氢材料 Photocatalytic materials for solar hydrogen from water splitting • 1.光催化制氢基本原理 • 2.光催化制氢反应体系 • 3.光催化制氢材料
查看元素周期表! Periodic Table of the Elements,in Pictures Key Atoms .C H Atomic Atoms have a nucleus of Number Color Key Name Carbon protons and neutrons The atomic AkaM以as surrounded by electrons. number is Alkal Earth Metals 3 The number of electrons in an Carbon the numaet Boron Nitrogen Oxygen Transition Metals ●proton uncharged atom is the same as Grouo Group Group Group Halogens Balloons Use or Plants and of protons Occurrence Animals in an atom Other Metals electron the number of protons. 0 9 Ne 10 Other Non-Metals Boron Carbon Nitrogen Fluorine Neon Halogens Molecules Solid OPEN at room Inert Gases Watch Liquid ①⊙⊙ Atoms combine to make temperature molecules by sharing or trading at-Res istant Pan零anG Batteries Emeralds Canthanides 的Gas their outer electrons. Glassware Animals Toothpaste Signs Actinides Na 11 M12 A13 D C317Ar18 Sodium Radioactive Many atoms prefer to have ⊙15S16 Trans-Actinides con Phosphorus Sulphur Man-Made eight electrons in their outer orbt H20 lke the oxygen atom in H2O. Baking Green Rocks Soda T A N 0 M E A L Foil Sand,Dirt Bones Eog Yolks 日leach Light Bulbs 19 Ca20 21Ti 022 23 Cr 24 Mn 25 Fe 26 Co 27Ni 28 Cu 29 Zn 30 Ga 31 Ge 32 As 33 Se 34 Br 635Kr36 Potassium Calcium Scandum Titanium /anadiu Chromium Manganesel Iron Cobalt Nickel Copper Zinc Gallum Germanium Arsenic Selenlum Krypton 2:00 ROCK Electric Light-Emiting Fertilizer Teeth Springs Car Trim Crushers Buildings Magnets Coins Vr色 日ras5 Dodes 日ectronics Poison Solar Cels Sedatives L自。营 Rb 37 38 39 40 Nb 41Mo 42 43Ru 44 Rh 15 Pd 46 Ag 47 Cd 8 n 49 Sn 50 Sb 51 Te 52 53 enium ellurium Zrcon Mag Lev PoDutior Solar Cells Gems Irain Tools U川an0ss Contacts Crucibles Control Batteries Electronics Cans Type Metal Solar Cels Antiseptic Lighthouses Cs255 56 72 Ta 74 Re 750s 761r 77Pt278 Au 79Hg0 0 TI 81 Pb 82 83 Po 84 At 85 86 Osmum nidum Plaunum Golc Mercury Lead 日ismh 1200001 See Below Atomic Nuclear FIre Anti-Static X-Ravs Comrol Joms Light Bubs Light Bulbs Pen Points Meteor Jewelry Jewelry Thermoneters Poison Weights Sprinklers Brushes Short-Lived Therapy Fr 87 1040b 105 Sg 106Bh 107Hs 08t 109 Uun 110 Uuu 111 Uub 112 Uut Radum 113 Uuq 114 445hh 116 117 118 nhe um See Below Few Uses Luminous Short-Lived Paint Groups tTrans-Actinides: nt 112 was the highest-numbered element yet created,as of 1996. La 57 Ce H2+02>H20 66 Ho 67Er 68 Tm 69Yb 70 Lu 71 The vertical columns anthanide Lutetium Color Coor Color Color Flints Power Lamps Phosphors Lasers Phosphors Phosphors Phosphors 89 Pa 91U92Hp93Pu94Am95 Cm 96 Bk 97 99 Fm 100 Md101 No 2102 L 103 ranium 上海气通大粤 SHANGHAI JLAO TONG UNIVERSTTY Short-Lived Shdrt-Lived irt-Lived ort-Liveo source Very Rare Power Detectors Weapons Power Gauges (Months) IDaYs (Hours) (Minutes) rseconds
查看元素周期表! H H2 + O2 → H2O

氢能源的主要特点 宫8 资源丰富 1020kg(在水中)(H20=H2+ 02) ●热量大 1kg氢气=2.7kg汽油=3.54kg煤 ●没有污染:H2+O2=H20 ●可以利用原有基础设施
氢能源的主要特点 • 资源丰富 1020 kg (在水中) (H2O = H2 + O2) 热量大 1kg 氢气 = 2.7kg 汽 油 = 3.54kg 煤 没有污染: H2 + O2 = H2O 可以利用原有基础设施