
CHAPTER 7.HAZARD CHARACTERIZATION 危害特征描述

COMPONENTS OF RISK ASSESSMENT Hazard Identification Dose-Response Assessment Exposure Assessment Risk Characterization
Hazard Identification Dose-Response Assessment Exposure Assessment Risk Characterization

DEFINITION The qualitative and/or quantitative evaluation of the nature of the adverse health effects associated with biological,chemical and physical agents which may be present in food.For chemical agents,a dose-response assessment should be performed.For biological or physical agents,a dose-response assessment should be performed if the data are obtainable. +有害作用属性的定性或定量评价 +化学危害的剂量-反应评估 +微生物或物理危害有条件下的的剂量-反应评估
The qualitative and/or quantitative evaluation of the nature of the adverse health effects associated with biological, chemical and physical agents which may be present in food. For chemical agents, a dose-response assessment should be performed. For biological or physical agents, a dose-response assessment should be performed if the data are obtainable. 有害作用属性的定性或定量评价 化学危害的剂量-反应评估 微生物或物理危害有条件下的的剂量-反应评估

DOSE-RESPONSE x"All substances are poisons;there is none which is not a poison.The right dose differentiates a poison from a remedy." Paracelsus (1493-1541)
“All substances are poisons; there is none which is not a poison. The right dose differentiates a poison from a remedy.” Paracelsus (1493-1541)
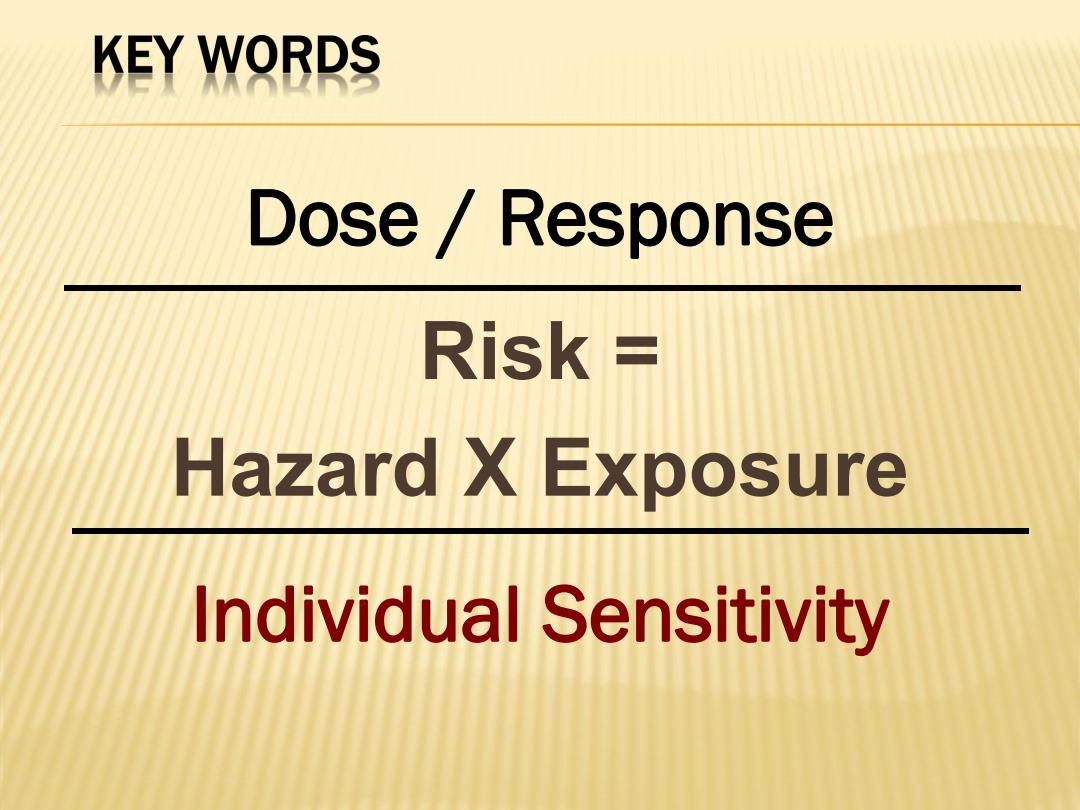
KEY WORDS Dose Response Risk Hazard X Exposure Individual Sensitivity
Risk = Hazard X Exposure Dose / Response Individual Sensitivity

EFFECTS OF AMOUNT ON RESPONSE 的
EFFECTS OF SIZE ON RESPONSE
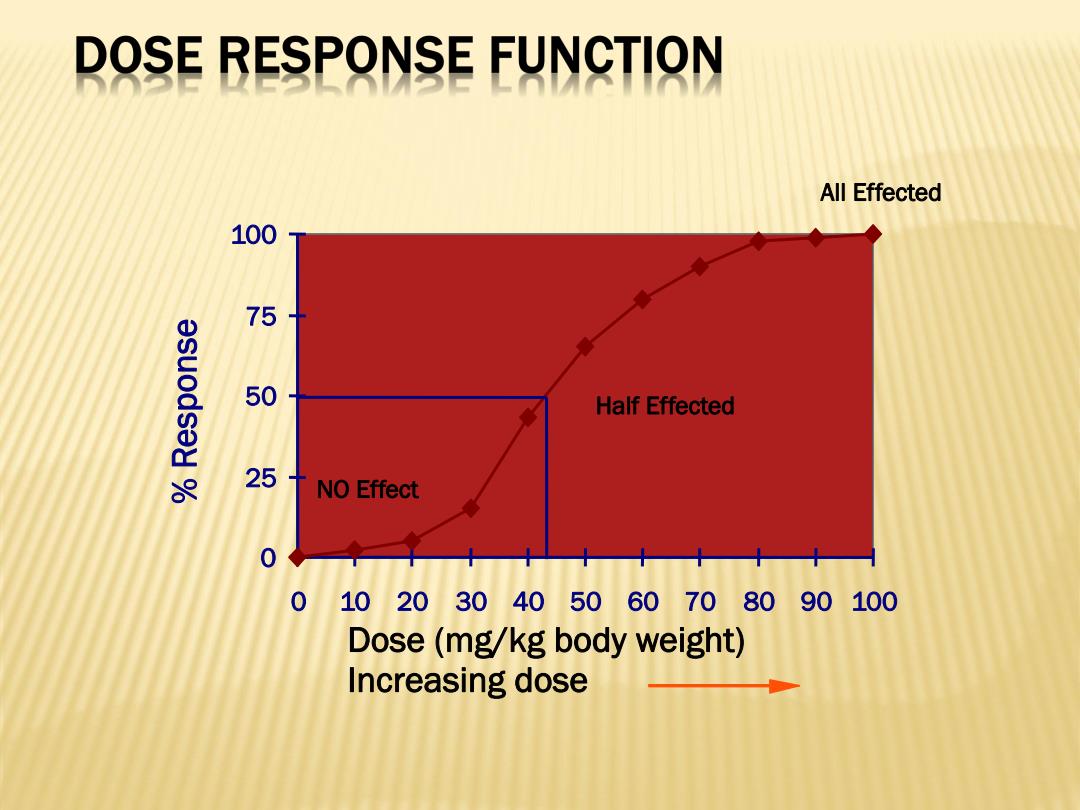
DOSE RESPONSE FUNCTION All Effected 100 75 50 Half Effected 25十 NO Effect 102030405060708090100 Dose(mg/kg body weight) Increasing dose
Dose (mg/kg body weight) Increasing dose 0 25 50 75 100 0 10 20 30 40 50 60 70 80 90 100 % R e s p o n s e NO Effect All Effected Half Effected

PARAMETERS TO EVALUATE TOXICITY Half lethal dose(LDso):给予单次剂量的受试物后,预期引起半数实验 动物死亡的剂量水平 Maximal tolerance dose(MTD):不致引起受试动物死亡的最大剂量 Threshold dose,Lowest observed adverse effect level (LOAEL): 诱发机体某种生物效应呈现的最低剂量 No observed adverse effect level(NOAEL):用现有的技术手段和指 标未观察到外源物对受试机体产生毒性效应的最大剂量或浓度 Reference dose(RfD):每日暴露的毒物不至于对人群健康产生有害影 响的剂量水平,由美国环保局提出,用于非致癌性物质进行风险评价 Benchmark dose(BMD):依据动物试验剂量-反应关系的结果,用一定 的统计学模型求得的受试机体引起一定比例(定量资料为10%,定性资料 为5%)动物出现阳性反应剂量的95%可信限区间下限值,用于发育毒性和 生殖毒性的风险评价
Ø Half lethal dose (LD50 ):给予单次剂量的受试物后,预期引起半数实验 动物死亡的剂量水平 Ø Maximal tolerance dose (MTD):不致引起受试动物死亡的最大剂量 Ø Threshold dose, Lowest observed adverse effect level (LOAEL): 诱发机体某种生物效应呈现的最低剂量 Ø No observed adverse effect level (NOAEL):用现有的技术手段和指 标未观察到外源物对受试机体产生毒性效应的最大剂量或浓度 Ø Reference dose (RfD):每日暴露的毒物不至于对人群健康产生有害影 响的剂量水平,由美国环保局提出,用于非致癌性物质进行风险评价 Ø Benchmark dose (BMD):依据动物试验剂量-反应关系的结果,用一定 的统计学模型求得的受试机体引起一定比例(定量资料为10%,定性资料 为5%)动物出现阳性反应剂量的95%可信限区间下限值,用于发育毒性和 生殖毒性的风险评价
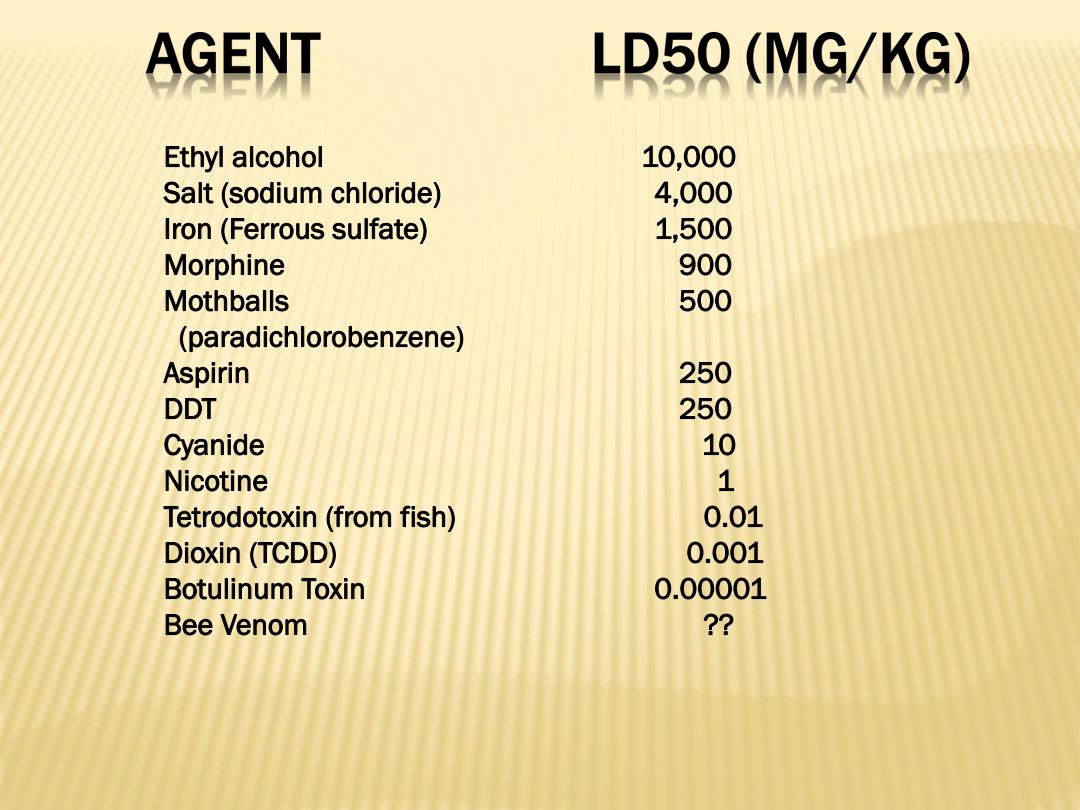
AGENT LD50(MG/KG) Ethyl alcohol 10,000 Salt(sodium chloride) 4,000 Iron(Ferrous sulfate) 1,500 Morphine 900 Mothballs 500 (paradichlorobenzene) Aspirin 250 DDT 250 Cyanide 10 Nicotine 1 Tetrodotoxin(from fish) 0.01 Dioxin (TCDD) 0.001 Botulinum Toxin 0.00001 Bee Venom ?
Ethyl alcohol 10,000 Salt (sodium chloride) 4,000 Iron (Ferrous sulfate) 1,500 Morphine 900 Mothballs 500 (paradichlorobenzene) Aspirin 250 DDT 250 Cyanide 10 Nicotine 1 Tetrodotoxin (from fish) 0.01 Dioxin (TCDD) 0.001 Botulinum Toxin 0.00001 Bee Venom ??