Physical and Chemical Properties of Materials) 课程教学大纲 一、课程基本信息 英文名称 Physical and Chemical Properties 课程代码 MDNE2004 of Materials 课程性质Major Basic 授课对象 New Energy Science and Engineering 学分4 学时 72h 主讲载师陈煜、耿凤霞 修订日期 2021.8.19 Materials 2.An Introduction to 二、课程目标 (一)总体目标: This course aims to present the basic fundamentals of materials properties on an appropriate level for university students with certain knowledge on chemistry and physics. (二)课程目标 Important topics,including atomic structure and interatomic bonding.the structure of crystalline solids,imperfections in solids,diffusion,mechanical properties of metals,dislocations and strengthening mechanisms,phase diagrams,phase transformations,electrical properties, amo Understanding imporant conept 三、教学内容 Chapter1:Introduction
《Physical and Chemical Properties of Materials》 课程教学大纲 一、课程基本信息 英文名称 Physical and Chemical Properties of Materials 课程代码 MDNE2004 课程性质 Major Basic 授课对象 New Energy Science and Engineering 学 分 4 学 时 72 h 主讲教师 陈煜、耿凤霞 修订日期 2021.8.19 指定教材 M. F. Ashby, D. R. H. Jones, Engineering Materials 2, An Introduction to Microstructures, Processing and Design, Third Edition, Elsevier, 2006. 二、课程目标 (一)总体目标: This course aims to present the basic fundamentals of materials properties on an appropriate level for university students with certain knowledge on chemistry and physics. (二)课程目标: Important topics, including atomic structure and interatomic bonding, the structure of crystalline solids, imperfections in solids, diffusion, mechanical properties of metals, dislocations and strengthening mechanisms, phase diagrams, phase transformations, electrical properties, thermal properties, and optical properties are among discussion. Understanding important concepts and solving problems with formulas are emphasized in this course. 三、教学内容 Chapter 1: Introduction Contents: 1.1 Historical Perspective 1.2 Physical And Chemical Properties Of Materials 1.3 Why Study The Properties Of Materials? 1.4 Classification Of Materials 1.5 Advanced Materials 1.6 Modern Materials’ Needs

Problems: 1.Within this classroom,find one example for each of the categories (ie.,metal,ceramic,and polymer). Chapter 2:Atomic Structure and Interatomic Bonding 22 Fundamental Concepts 2.5 Bonding Forces And Energies oiacgpydoesthsadhesveapea创snaheaceadngrar 2.Why are the atomic weights ofel s generally not integers? than metalical bonded ones. Chapter 3:The Structure of Crystalline Solids Satam Fudamemtal Concept 3.6 Polymorphism And Allotropy 3.9Crystallographic Directions And sities 3.12 Close-Packed Crystal Structures 315 Anisot 3.1 D eeion Of Cysal ucture 3.Copper has an atomic radiusof.FCCcrystal structure,and an atomic weight of63.5 1.Compute its th Chapter4:Imperfections in Solids 42 Vacancies And Self-Interstitials
Problems: 1、Within this classroom, find one example for each of the categories (i.e., metal, ceramic, and polymer). Chapter 2: Atomic Structure and Interatomic Bonding Contents: 2.1 Introduction 2.2 Fundamental Concepts 2.3 Electrons In Atoms 2.4 The Periodic Table 2.5 Bonding Forces And Energies 2.6 Primary Interatomic Bonds 2.7 Secondary Bonding Or Van Der Waals Bonding 2.8 Molecules Problems: 1. What category does this adhesive tape fall as an advanced material? 2. Why are the atomic weights of elements generally not integers? 3. Give electron configurations for the Fe3+ and S2- ions. 4. Explain why covalently bonded materials are generally less dense than ionically or metallically bonded ones. Chapter 3: The Structure of Crystalline Solids Contents: 3.1 Introduction 3.2 Fundamental Concepts 3.3 Unit Cells 3.4 Metallic Crystal Structures 3.5 Density Computations 3.6 Polymorphism And Allotropy 3.7 Crystal Systems 3.8 Point Coordinates 3.9 Crystallographic Directions 3.10 Crystallographic Planes 3.11 Linear And Planar Densities 3.12 Close-Packed Crystal Structures 3.13 Single Crystals 3.14 Polycrystalline Materials 3.15 Anisotropy 3.16 X-Ray Diffraction: Determination Of Crystal Structures 3.17 Noncrystalline Solids Problems: 1. Calculate the volume of an FCC unit cell in terms of the atomic radius R. 2. Prove that the atomic packing factor (APF) for FCC is 0.74. 3. Copper has an atomic radius of 0.128 nm, an FCC crystal structure, and an atomic weight of 63.5 g/mol. Compute its theoretical density and compare the answer with its measured density. 4. For cubic crystals, as values of the planar indices h, k, and l increase, does the distance between adjacent and parallel planes (i.e., the interplanar spacing) increase or decrease? Why? Chapter 4: Imperfections in Solids Contents: 4.1 Introduction 4.2 Vacancies And Self-Interstitials

4 Bulk 4 Basic Concepts of Microscopy Pmobkcn pletesolid solubility may ccur for substitutional solid solutions but not for y fa sinle cyds ontDe h decreasing grain size?Why? Chapter 5:Diffusion Contents: .3 Steady-State Diffu S.4 Nonst eady-Sta Di伍 56 Diffusion In Semic 5.7 Other Diffusion Paths Problems 1.Consider the self-diffu Ca(B) 171,plot (and abel)lines h metals given t Chapter6:Mechanical Properties of Metal Contents: 6)lntroductior ss And Strain ties Of Materials 6.7True Stress And Strain 69c And nations 6.10 Hardness 6.11 Variability Of Material Properties Problems: alloy to the point of fracture.Now superimpose on this plot a schematic compressive stress-strain curve for the same alloy.Explain any differences between the two curves
4.3 Impurities In Solids 4.4 Specification Of Composition 4.5 Dislocations—Linear Defects 4.6 Interfacial Defects 4.7 Bulk Or Volume Defects 4.8 Atomic Vibrations 4.9 Basic Concepts Of Microscopy 4.10 Microscopic Techniques 4.11 Grain Size Determination Problems: 1. Explain why complete solid solubility may occur for substitutional solid solutions but not for interstitial solid solutions. 2. The surface energy of a single crystal depends on crystallographic orientation. Does this surface energy increase or decrease with an increase in planar density? Why? 3. Does the grain size number increase or decrease with decreasing grain size? Why? Chapter 5: Diffusion Contents: 5.1 Introduction 5.2 Diffusion Mechanisms 5.3 Steady-State Diffusion 5.4 Nonsteady-State Diffusion 5.5 Factors That Influence Diffusion 5.6 Diffusion In Semiconducting Materials 5.7 Other Diffusion Paths Problems: 1. Consider the self-diffusion of two hypothetical metals A and B. On a schematic graph of ln D versus 1/T, plot (and label) lines for both metals given that D0(A) > D0(B) and also that Qd(A) > Qd(B). Chapter 6: Mechanical Properties of Metals Contents: 6.1 Introduction 6.2 Concepts Of Stress And Strain 6.3 Stress–Strain Behavior 6.4 Anelasticity 6.5 Elastic Properties Of Materials 6.6 Tensile Properties 6.7 True Stress And Strain 6.8 Elastic Recovery After Plastic Deformation 6.9 Compressive, Shear, And Torsional Deformations 6.10 Hardness 6.11 Variability Of Material Properties Problems: 1. Cite the primary differences between elastic, anelastic, and plastic deformation behaviors. 2. Make a schematic plot showing the tensile engineering stress–strain behavior for a typical metal alloy to the point of fracture. Now superimpose on this plot a schematic compressive engineering stress–strain curve for the same alloy. Explain any differences between the two curves

Chapter7:Dislocations and Strengthening Mechanisms 7.3 as 7.4 Slip Systems iofDisloecaioms Slip In ingle Crystals fPolycrystalline Materials Strengthening By GrainSiz e Reduction 7.11 Recover 7.13 Grain Problems the di 2 Would you ex pect a crystalline ceramic material to strain harden at room temperature?Why or why no xplain why some metals(and tin)do not strain harden when deformed at room tem epeeme merey why not? Chapter8:Phase Diagrams 82Solubility Limit hases Phase Equilibria On-Component (Or Unary)Phase Diagrams ams 8.9 Development Of Microstructure In Isomor es o 8.12 Development Of Microstructure In Eutectic Alloys 1 Equilib Having Intermediate Phases Or Compounds 8.15 Congruent Phase Transformations yPhase Diagrams 8.18 The Iron-Iron Carbide(Fe-Fee)Phase Diagram Problems: in Cu? 3.Briefly explain why a proeutectoid phase (ferite or cementite)forms along austenite grain boundaries
Chapter 7: Dislocations and Strengthening Mechanisms Contents: 7.1 Introduction 7.2 Basic Concepts 7.3 Characteristics Of Dislocations 7.4 Slip Systems 7.5 Slip In Single Crystals 7.6 Plastic Deformation Of Polycrystalline Materials 7.7 Deformation By Twinning 7.8 Strengthening By Grain Size Reduction 7.9 Solid-Solution Strengthening 7.10 Strain Hardening 7.11 Recovery 7.12 Recrystallization 7.13 Grain Growth Problems: 1. Explain the difference between resolved shear stress and critical resolved shear stress. 2. Would you expect a crystalline ceramic material to strain harden at room temperature? Why or why not? 3. Briefly explain why some metals (i.e., lead and tin) do not strain harden when deformed at room temperature. 4. Would you expect it to be possible for ceramic materials to experience recrystallization? Why or why not? 5. Would you expect it to be possible for ceramic materials to experience recrystallization? Why or why not? Chapter 8: Phase Diagrams Contents: 8.1 Introduction 8.2 Solubility Limit 8.3 Phases 8.4 Microstructure 8.5 Phase Equilibria 8.6 One-Component (Or Unary) Phase Diagrams 8.7 Binary Isomorphous Systems 8.8 Interpretation Of Phase Diagrams 8.9 Development Of Microstructure In Isomorphous Alloys 8.10 Mechanical Properties Of Isomorphous Alloys 8.11 Binary Eutectic Systems 8.12 Development Of Microstructure In Eutectic Alloys 8.13 Equilibrium Diagrams Having Intermediate Phases Or Compounds 8.14 Eutectoid And Peritectic Reactions 8.15 Congruent Phase Transformations 8.16 Ceramic And Ternary Phase Diagrams 8.17 The Gibbs Phase Rule 8.18 The Iron–Iron Carbide (Fe–Fe3c) Phase Diagram 8.19 Development Of Microstructure In Iron–Carbon Alloys 8.20 The Influence Of Other Alloying Elements Problems: 1. What is the difference between the states of phase equilibrium and metastability? 2. At 700oC (1290oF), what is the maximum solubility (a) of Cu in Ag? (b) Of Ag in Cu? 3. Briefly explain why a proeutectoid phase (ferrite or cementite) forms along austenite grain boundaries

eDevclopet of Microstructureandltertion of 93The Kinetics Of Phase Transformations tastable vers ms Mechanic or Of Iron-Carbon Alloys 9.9 Review Of Phase Transformations And Mechanical Properties For Iron-Carbon Alloys cooling heat treatment procedure that would be used to that ould be required to produce a specimen having a hardness of 93 HRB. Chapter 10:Electrical Properties Commodaedion 10.2Ohm's Law l03Elctrical.cComtctivity tomic Bonding Models 10.Electrical Resistivity Of Metals Affect Carrier Mobility aterials ce Of The Dielectric Constant rength Dielectric Materials 10.25 Piezoelectricity Problems 1.If a metallic material is cooled through its melting temperature at an extremely rapid rate,it will form a noncrystalline solid (ie a metallic glass)Will the l conductivity of the ow as dopant level is increased would you expect the temperature at
Chapter 9: Phase Transformations: Development of Microstructure and Alteration of Mechanical Properties Contents: 9.1 Introduction 9.2 Basic Concepts 9.3 The Kinetics Of Phase Transformations 9.4 Metastable Versus Equilibrium States 9.5 Isothermal Transformation Diagrams 9.6 Continuous Cooling Transformation Diagrams 9.7 Mechanical Behavior Of Iron–Carbon Alloys 9.8 Tempered Martensite 9.9 Review Of Phase Transformations And Mechanical Properties For Iron–Carbon Alloys Problems: 1. Which is more stable, the pearlitic or the spheroiditic microstructure? Why? 2. Briefly describe the simplest continuous cooling heat treatment procedure that would be used to convert a 4340 steel from (martensite + bainite) to (ferrite + pearlite). 3. For a eutectoid steel, describe an isothermal heat treatment that would be required to produce a specimen having a hardness of 93 HRB. Chapter 10: Electrical Properties Contents: 10.1 Introduction 10.2 Ohm’s Law 10.3 Electrical Conductivity 10.4 Electronic And Ionic Conduction 10.5 Energy Band Structures In Solids 10.6 Conduction In Terms Of Band And Atomic Bonding Models 10.7 Electron Mobility 10.8 Electrical Resistivity Of Metals 10.9 Electrical Characteristics Of Commercial Alloys 10.10 Intrinsic Semiconduction 10.11 Extrinsic Semiconduction 10.12 The Temperature Dependence Of Carrier Concentration 10.13 Factors That Affect Carrier Mobility 10.14 The Hall Effect 10.15 Semiconductor Devices 10.16 Conduction In Ionic Materials 10.17 Electrical Properties Of Polymers 10.18 Capacitance 10.19 Field Vectors And Polarization 10.20 Types Of Polarization 10.21 Frequency Dependence Of The Dielectric Constant 10.22 Dielectric Strength 10.23 Dielectric Materials 10.24 Ferroelectricity 10.25 Piezoelectricity Problems: 1. If a metallic material is cooled through its melting temperature at an extremely rapid rate, it will form a noncrystalline solid (i.e., a metallic glass).Will the electrical conductivity of the noncrystalline metal be greater or less than its crystalline counterpart? Why? 2. Will Zn act as a donor or acceptor when added to the compound semiconductor GaAs? Why? (Assume that Zn is a substitutional impurity.) 3. On the basis of figure below, as dopant level is increased would you expect the temperature at

whichasemouctor becomes ininictoinrease toremain essentially the samertoerease? increasing temperature to influence the operation of pnjunction rectifiers and Chapter 11:Thermal Properties Contents duction 12 Heat Cap 11.3 Thermal Exp Proble (a) lain a glas (b)Supp Wh al conductivity of a plain carbon steel is greater than for a stainless steel.Why is this The the ermal cond feels cole to the touch than a plastic steering wheel,even though both are at the same temperature. Chapter 12:Optical Properties Conter And Elns Wit olid 12.5 Refraction Problems diemuee the ei milarities and differ 2.Electromagnetic radiation may be treated from the classical or the quantum-mechanical o viewpoints 4.Which of the follow N.Mgo?Why? Compare th e the of metals and transparent nonmetal 四、学时分配
which a semiconductor becomes intrinsic to increase, to remain essentially the same, or to decrease? Why? 4. Would you expect increasing temperature to influence the operation of p–n junction rectifiers and transistors? Explain Chapter 11: Thermal Properties Contents: 11.1 Introduction 11.2 Heat Capacity 11.3 Thermal Expansion 11.4 Thermal Conductivity 11.5 Thermal Stresses Problems: 1. (a) Explain why a brass lid ring on a glass canning jar will loosen when heated. (b) Suppose the ring is made of tungsten instead of brass. What will be the effect of heating the lid and jar? Why? 2. The thermal conductivity of a plain carbon steel is greater than for a stainless steel. Why is this so? 3. The thermal conductivity of a single-crystal ceramic specimen is slightly greater than a polycrystalline one of the same material. Why is this so? 4. Explain why, on a cold day, the metal door handle of an automobile feels colder to the touch than a plastic steering wheel, even though both are at the same temperature. Chapter 12: Optical Properties Contents: 12.1 Introduction 12.2 Electromagnetic Radiation 12.3 Light Interactions With Solids 12.4 Atomic And Electronic Interactions 12.5 Refraction 12.6 Reflection 12.7 Absorption 12.8 Transmission 12.9 Color 12.10 Opacity And Translucency In Insulators 12.11 Luminescence 12.12 Photoconductivity 12.13 Lasers 12.14 Optical Fibers In Communications Problems: 1. Briefly discuss the similarities and differences between photons and phonons. 2. Electromagnetic radiation may be treated from the classical or the quantum-mechanical perspective. Briefly compare these two viewpoints. 3. Why are metals transparent to high-frequency x-ray and γ-ray radiation? 4. Which of the following oxide materials when added to fused silica (SiO2) will increase its index of refraction: Al2O3, TiO2, NiO, MgO? Why? 5. Compare the factors that determine the characteristic colors of metals and transparent nonmetals. 四、学时分配
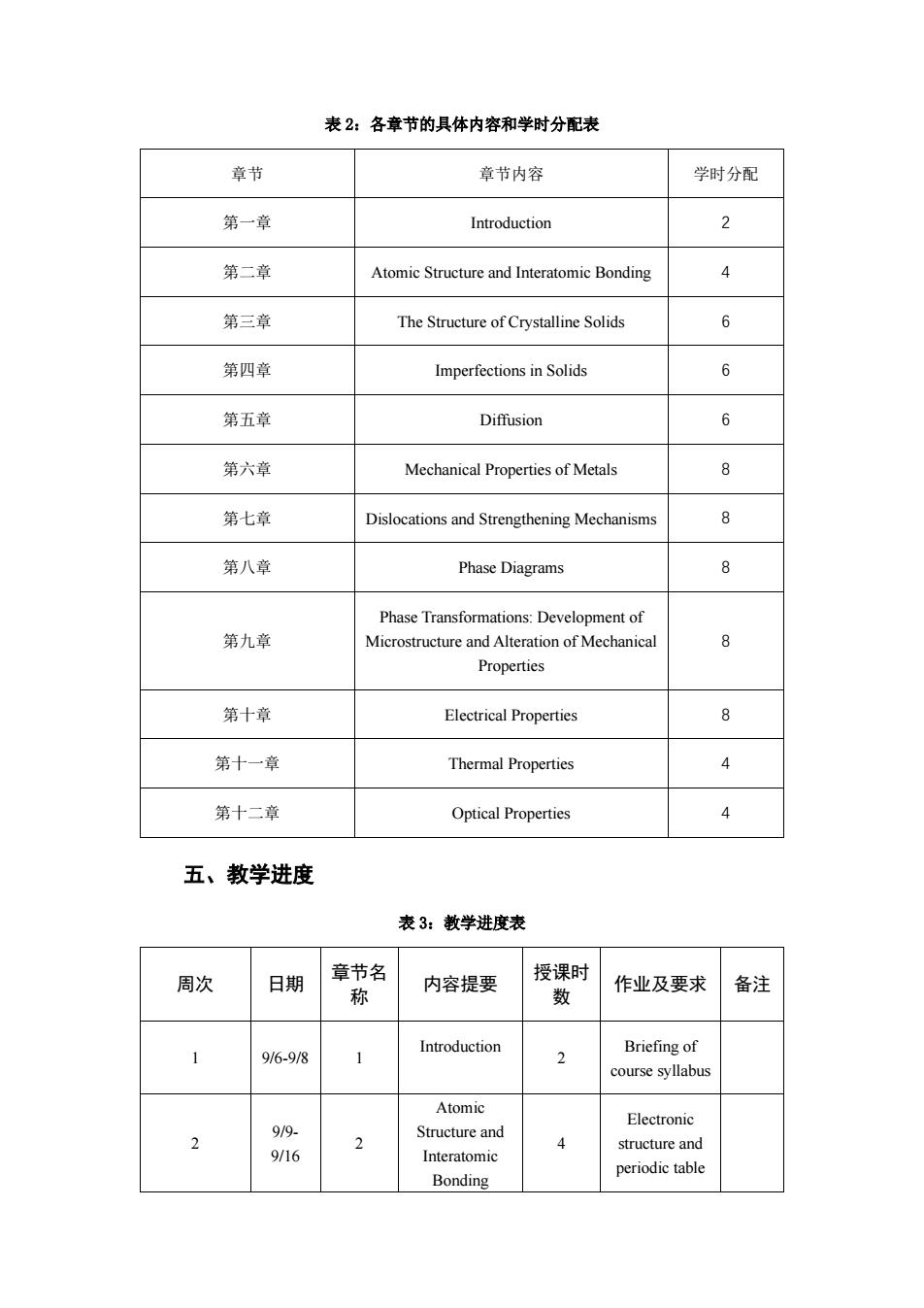
表2:各章节的具体内容和学时分配表 章节 章节内容 学时分配 第一章 Introduction 第二章 Atomic Structure and Interatomic Bonding 第三章 The Structure of Crystalline Solids 6 第四章 Imperfections in Solids 6 第五章 Diffusion 6 第六章 Mechanical Properties of Metals 第七章 Dislocations and Strengthening Mechanisms 8 第八章 Phase Diagrams Phase Transformations:Development of 第九章 Microstructure and Alteration of Mechanica 8 Properties 第十章 Electrical Properties 第十一章 Thermal Properties 4 第十二章 Optical Properties 五、教学进度 表3:教学进度表 周次 日期 名 内容提要 作业及要求 备注 1 916-9/8 Introduction Briefing of 2 course syllabus Atomic 2 9/9. Electronic Structure and 4 structure and 9/16 Interatomic Bonding periodic table
表 2:各章节的具体内容和学时分配表 章节 章节内容 学时分配 第一章 Introduction 2 第二章 Atomic Structure and Interatomic Bonding 4 第三章 The Structure of Crystalline Solids 6 第四章 Imperfections in Solids 6 第五章 Diffusion 6 第六章 Mechanical Properties of Metals 8 第七章 Dislocations and Strengthening Mechanisms 8 第八章 Phase Diagrams 8 第九章 Phase Transformations: Development of Microstructure and Alteration of Mechanical Properties 8 第十章 Electrical Properties 8 第十一章 Thermal Properties 4 第十二章 Optical Properties 4 五、教学进度 表 3:教学进度表 周次 日期 章节名 称 内容提要 授课时 数 作业及要求 备注 1 9/6-9/8 1 Introduction 2 Briefing of course syllabus 2 9/9- 9/16 2 Atomic Structure and Interatomic Bonding 4 Electronic structure and periodic table
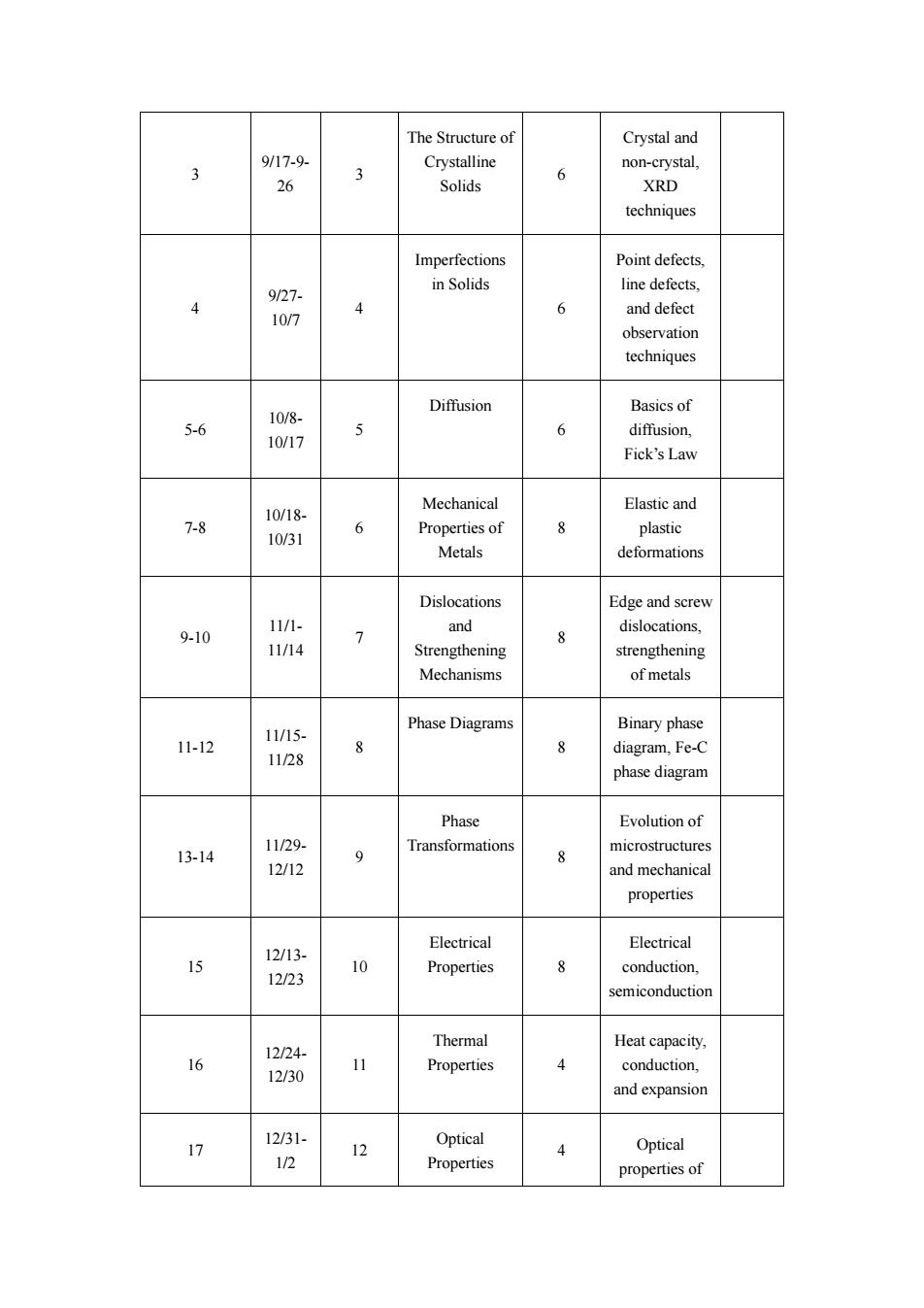
The Structure of Crystal and 9/17-9 3 6 non-crystal Solids XRD techniques Point defects 927. in Solids line defect 4 6 10/7 and defect observation techniques 10/8- Diffusion Basicsof 5-6 10/17 5 6 diffusion, Fick's Law Mechanical Elastic and 7-8 6 8 plastic Metals deformations Dislocations Edge and screv 9-10 11/1- > and dislocations Strengthening 8 strengthening Mechanisms of metals Phase Diagrams Binary phase 11/15. 1-2 1128 8 phase diagram Phase Evolution of 13-14 11/29- 12/12 9 Transformations microstructures properties Electrical Electrical 12/13- 12/23 10 Properties 8 conduction. Thermal Heat capacity, 16 12/24 12/30 Properties 4 conduction. and expansion 17 Optical Optical 1/2 4 Properties propertiesof
3 9/17 - 9 - 26 3 The Structure of Crystalline Solids 6 Crystal and non -crystal, XRD techniques 4 9/27 - 10/7 4 Imperfections in Solids 6 Point defects, line defects, and defect observation techniques 5 - 6 10/8 - 10/17 5 Diffusion 6 Basics of diffusion, Fick’s Law 7 - 8 10/18 - 10/31 6 Mechanical Properties of Metals 8 Elastic and plastic deformations 9 -10 11/1 - 11/14 7 Dislocations and Strengthening Mechanisms 8 Edge and screw dislocations, strengthening of metals 11 -12 11/15 - 11/28 8 Phase Diagrams 8 Binary phase diagram, Fe -C phase diagram 13 -14 11/29 - 12/12 9 Phase Transformations 8 Evolution of microstructures and mechanical properties 15 12/13 - 12/23 10 Electrical Properties 8 Electrical conduction, semiconduction 16 12/24 - 12/30 11 Thermal Properties 4 Heat capacity, conduction, and expansion 17 12/31 - 1/2 12 Optical Properties 4 Optical properties of

metals and non-metals 六、教材及参考书目 1.R T.KeHoff,Thermodynamics in Materials Science,McGraw-Hill,1993 2.D.A.Porter,K.E.Easterling.Phase Transformations in Metals and Alloys,Second Edition. CRCPress,1992 3.S.O.Kasap,Principles of Electronic Materials and Devices,Third Edition,McGraw-Hill, 2006. 4.K.Bowman.Mechanical Behavior of Materials,John Wiley&Sons,2003 5.A.Cottrell,An Introduction to Metallurgy,Second Edition,Edward Arnold,1975. 6.S.M.Allen,E.L.Thomas,The Structure of Materials,John Wiley&Sons,1999. 七、教学方法 1.讲授法 2.案例教学法 八、考核方式及评定方法 (一)课程考核与课程目标的对应关系 表4:课程考核与课程目标的对应关系表 课程目标 考核要点 考核方式 Understand the basic 课程目标1 fundamentals of materials In-class question-answering Midterm&Final Examination properties 课程目标2 Important contribution of Proiect Presentation Chinese Material Scientists (二)评定方法 1.评定方法 平时成绩:40%,期中考试:20%,期末考试40%
metals and non-metals 六、教材及参考书目 1. R. T. KeHoff, Thermodynamics in Materials Science, McGraw-Hill, 1993. 2.D. A. Porter, K. E. Easterling, Phase Transformations in Metals and Alloys, Second Edition, CRC Press, 1992. 3. S. O. Kasap, Principles of Electronic Materials and Devices, Third Edition, McGraw-Hill, 2006. 4. K. Bowman, Mechanical Behavior of Materials, John Wiley & Sons, 2003. 5. A. Cottrell, An Introduction to Metallurgy, Second Edition, Edward Arnold, 1975. 6. S. M. Allen, E. L. Thomas, The Structure of Materials, John Wiley & Sons, 1999. 七、教学方法 1.讲授法 2.案例教学法 八、考核方式及评定方法 (一)课程考核与课程目标的对应关系 表 4:课程考核与课程目标的对应关系表 课程目标 考核要点 考核方式 课程目标 1 Understand the basic fundamentals of materials properties In-class question-answering, Midterm & Final Examination 课程目标 2 Important contribution of Chinese Material Scientists Project & Presentation (二)评定方法 1.评定方法 平时成绩:40%,期中考试:20%,期末考试 40%