
热身活动:抽卡 1 2 4 5 6 7 8
1 2 3 4 热身活动:抽卡 5 6 7 8 严禁复制

热身活动:奔现的心情如何? (抽卡+弹幕分享) 1了土大文天关大 它反映出你来到线下 1 2 3 课堂的什么心情? 此刻你有什么想法? 5 6 7 8
1 2 3 4 热身活动:奔现的心情如何?(抽卡+弹幕分享) 它反映出你来到线下 课堂的什么心情? 此刻你有什么想法? 5 6 7 8 严禁复制
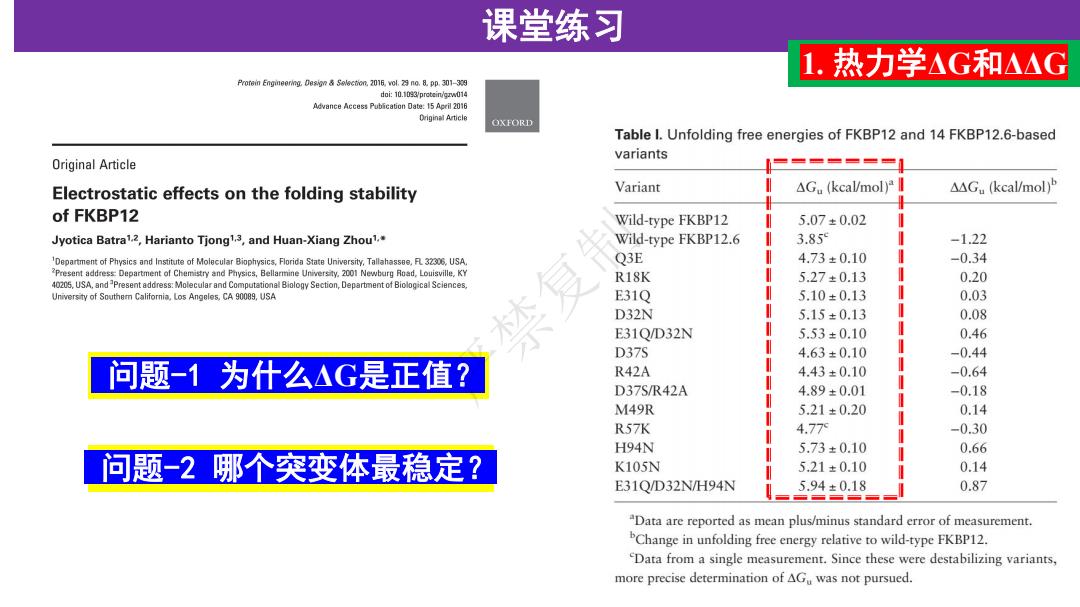
课堂练习 1.热力学△G和△△G Prorein Engineering.Solection.2016,vol.29 no.8.pp.301-339 doi:10.1093/protein/gzw014 Advance Access Pubfcation Date:15 April 2016 Driginal Article OXFORD Table I.Unfolding free energies of FKBP12 and 14 FKBP12.6-based variants Original Article Electrostatic effects on the folding stability Variant △G.(kcal/mol)a △△G.(kcal/mol)b of FKBP12 Wild-type FKBP12 5.07±0.02 Jyotica Batra1.2,Harianto Tjong1.3,and Huan-Xiang Zhou1. Wild-type FKBP12.6 3.85 -1.22 Department of Physics and Institute of Molecular Biophysics,Florida State University.Tallahassee.R32306,USA Q3E 4.73±0.10 -0.34 Present address Department of Chemistry and Physics,Bellarmine University.2001 Newburg Road,Louisville,KY R18K 5.27±0.13 0.20 40205,USA,and Present address:Molecular and Computational Biology Section,Departent of Biological Sciences, University of Southem California.Los Angeles,CA 90089,USA E31Q 5.10±0.13 0.03 D32N 5.15±0.13 0.08 E31Q/D32N 5.53±0.10 0.46 D37S 4.63±0.10 -0.44 问题-1为什么△G是正值? R42A 4.43±0.10 -0.64 D37S/R42A 4.89±0.01 -0.18 M49R 5.21±0.20 0.14 R57K 4.77 -0.30 H94N 5.73±0.10 0.66 问题-2哪个突变体最稳定? K105N 5.21±0.10 0.14 E31Q/D32N/H94N 5.94±0.18 0.87 Data are reported as mean plus/minus standard error of measurement. Change in unfolding free energy relative to wild-type FKBP12. Data from a single measurement.Since these were destabilizing variants, more precise determination of AGu was not pursued
问题-2 哪个突变体最稳定? 课堂练习 1. 热力学ΔG和ΔΔG 问题-1 为什么ΔG是正值? 严禁复制
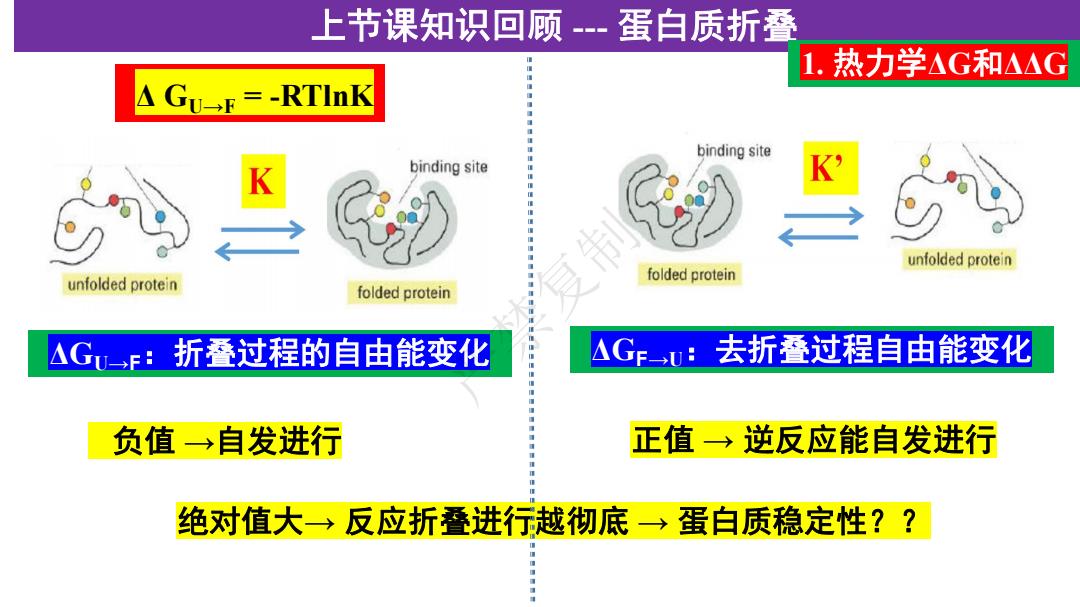
上节课知识回顾--蛋白质折叠 1.热力学AG和△△G △Gu→=-RTlnK binding site binding site unfolded protein unfolded protein folded protein folded protein △G→F:折叠过程的自由能变化 △GFu:去折叠过程自由能变化 负值→自发进行 正值→逆反应能自发进行 绝对值大→反应折叠进行越彻底→蛋白质稳定性??
上节课知识回顾 --- 蛋白质折叠 ΔGU→F:折叠过程的自由能变化 1. 热力学ΔG和ΔΔG 负值 →自发进行 绝对值大→ 反应折叠进行越彻底 → 蛋白质稳定性?? Δ GU→F = -RTlnK ΔGF→U:去折叠过程自由能变化 正值 → 逆反应能自发进行 严禁复制

课堂练习 1.热力学AG和△△G Prorein Engineering.Solection.2016,vol.29 no.8.pp.301-339 doi:10.1093/protein/gzw014 Advance Access Pubfcation Date:15 April 2016 Driginal Article OXFORD Table I.Unfolding free energies of FKBP12 and 14 FKBP12.6-based variants Original Article Electrostatic effects on the folding stability Variant △G.(kcal/mol)a △△G.(kcal/mol)P of FKBP12 Wild-type FKBP12 5.07±0.02 Jyotica Batra1.2,Harianto Tjong1.3,and Huan-Xiang Zhou1. Wild-type FKBP12.6 3.85 -1.22 Department of Physics and Institute of Molecular Biophysics,Florida State University.Tallahassee.R32306,USA Q3E 4.73±0.10 -0.34 Present address Department of Chemistry and Physics,Bellarmine University.2001 Newburg Road,Louisville,KY R18K 5.27±0.13 0.20 40205,USA,and Present address:Molecular and Computational Biology Section,Departent of Biological Sciences, University of Southem California.Los Angeles,CA 90089,USA E31Q 5.10±0.13 0.03 D32N 5.15±0.13 0.08 E31Q/D32N 5.53±0.10 0.46 D37S 4.63±0.10 -0.44 回答-1去折叠过程弘G正值 R42A 4.43±0.10 -0.64 D37S/R42A 4.89±0.01 -0.18 M49R 5.21±0.20 0.14 R57K 4.77 -0.30 H94N 5.73±0.10 0.66 ▣答-2E31Q/D32N/H94W K105N 5.21±0.10 0.14 E31Q/D32N/H94N 5.94±0.18 0.87 Data are reported as mean plus/minus standard error of measurement. Change in unfolding free energy relative to wild-type FKBP12. Data from a single measurement.Since these were destabilizing variants, more precise determination of AGu was not pursued
回答-2 E31Q/D32N/H94N 课堂练习 1. 热力学ΔG和ΔΔG 回答-1 去折叠过程ΔG正值 严禁复制
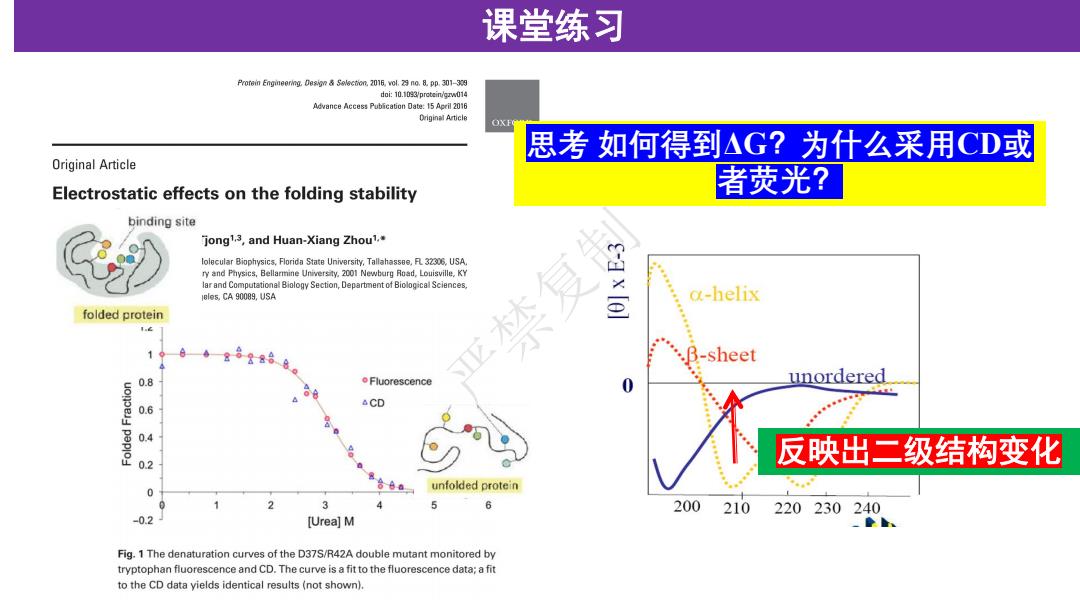
课堂练习 Prorein Engineering.Solection.2016,vol.29 no.8.pp.301-339 doi:10.1093/protein/gzw014 Advance Access Publication Date:15 Apeil 2016 Driginal Article OXE 思考如何得到△G?为什么采用CD或 Original Article Electrostatic effects on the folding stability 者荧光? binding site jong1.3,and Huan-Xiang Zhou1.* lolecular Biophysics,Florida State University,Tallahassee.R 32306,USA ry and Physics,Bellarmine University.2001 Newburg Road,Louisville,KY lar and Computational Biology Section,Department of Biological Sciences. eles.CA 90089.USA a-helix folded protein t.c 亚木 .B-sheet 0.8 o Fluorescence unordered △CD 0.6 0.4 反映出二级结构变化 0.2 unfolded protein 0 0 200 210 220230240 -0.2 [Urea]M Fig.1 The denaturation curves of the D37S/R42A double mutant monitored by tryptophan fluorescence and CD.The curve is a fit to the fluorescence data;a fit to the CD data yields identical results(not shown)
课堂练习 思考 如何得到ΔG?为什么采用CD或 者荧光? 反映出二级结构变化 严禁复制
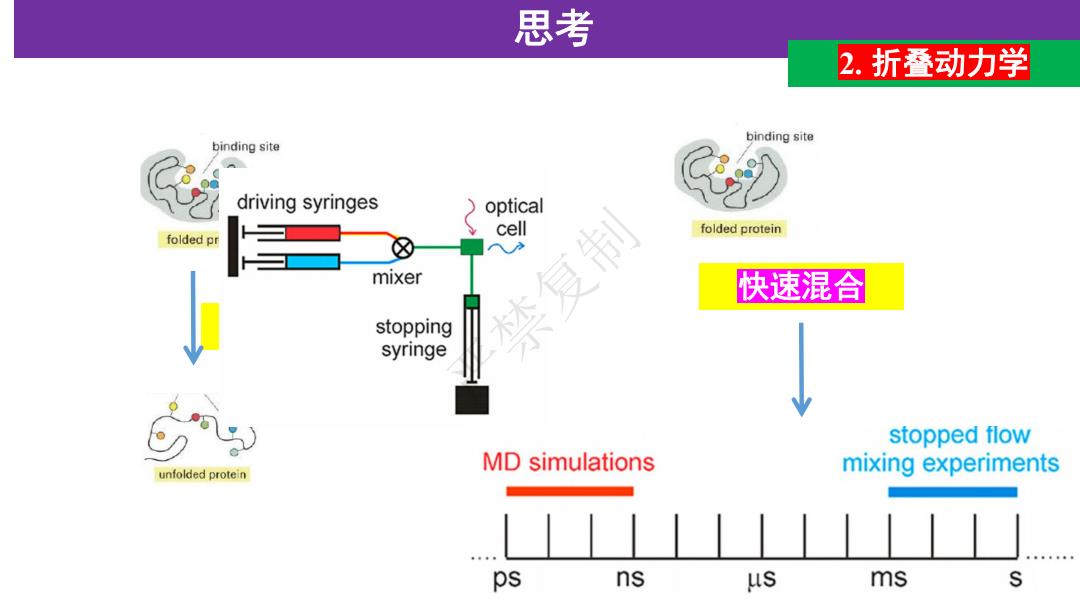
思考 2.折叠动力学 binding site binding site driving syringes optical folded pr 禁复制 cell folded protein 》 mixer 快速混合 stopping syringe stopped flow MD simulations unfolded protein mixing experiments ps ns us ms
盐酸胍诱导 思考 2. 折叠动力学 快速混合 圆二色信 号变化 Trp荧光 变化 严禁复制

课堂练习 2.折叠动力学 binding site binding site 0 -5 Tm =27 us 复制 10 15 ←Ta=136us 20 25 0 100 200300 400500600 Time (us) Trp荧光 unfolded protein 红外信号 变化 变化
T-jump 课堂练习 2. 折叠动力学 T-jump 红外信号 变化 Trp荧光 变化 严禁复制

课堂实验 的 yuwe
课堂实验 严禁复制

课堂实验结果 小组成员名字 02 血年 费贼阳 97 王氦 7 98 7 2, 罗洛成 98 指灵 98 9. 卢位 99 陈说 18 部敌☒ 刻限阳 98 5. 邓世涛 无泽 93
课堂实验结果 严禁复制