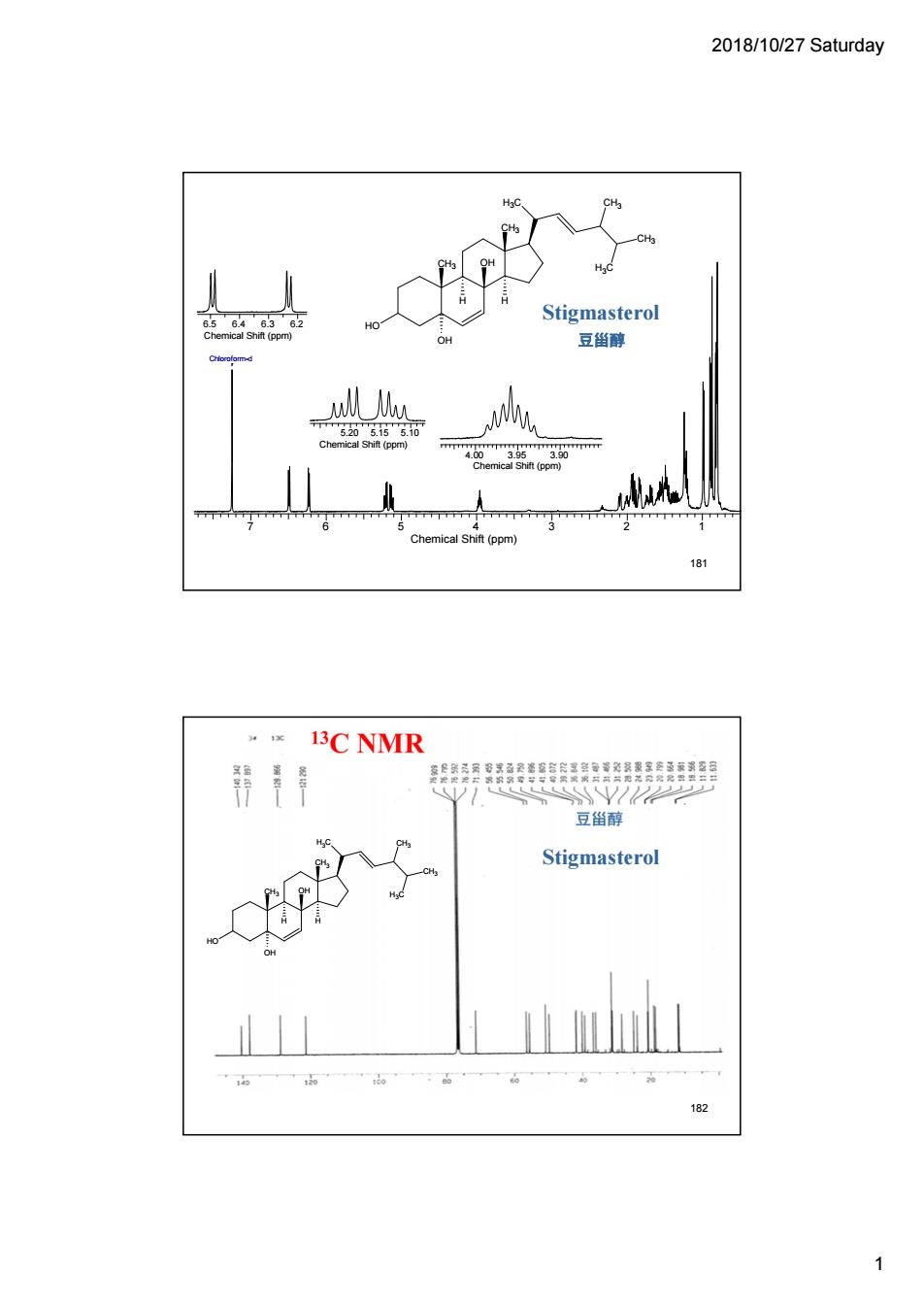
2018/10/27 Saturday Stigmasterol 豆甾醇 U mical i(pm 13C NMR 带月 豆甾醇 Stigmasterol 182 1
2018/10/27 Saturday 1 7 6 5 4 3 2 1 Chemical Shift (ppm) Chloroform-d 6.5 6.4 6.3 6.2 Chemical Shift (ppm) 5.20 5.15 5.10 Chemical Shift (ppm) 4.00 3.95 3.90 Chemical Shift (ppm) H3C HO CH3 CH3 OH H H CH3 CH3 H3C OH Stigmasterol 181 豆甾醇 H3C HO CH3 CH3 OH H H CH3 CH3 H3C OH Stigmasterol 182
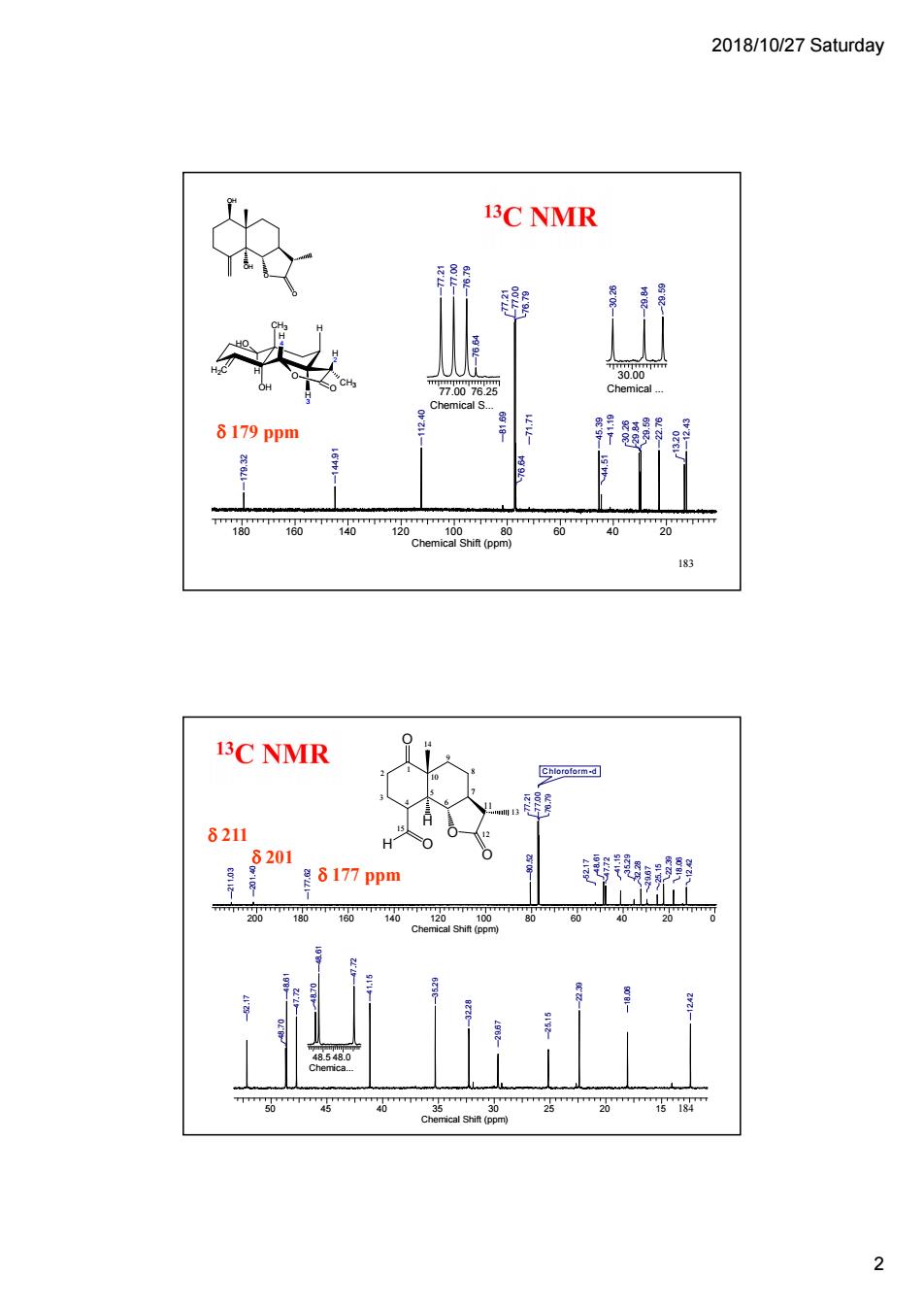
2018/10/27 Saturday a 3C NMR 7007为2 8179ppm 183 3C NMR 8211 是8177ppm c () 2
2018/10/27 Saturday 2 183 O OH OH O 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) 12.43 13.20 29.59 22.76 30.26 29.84 41.19 44.51 45.39 71.71 76.64 76.79 77.21 77.00 81.69 112.40 144.91 179.32 77.00 76.25 Chemical S... 76.64 77.21 77.00 76.79 30.00 Chemical ... 29.59 30.26 29.84 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 179 ppm 184 1 2 3 4 5 6 7 10 9 8 11 12 13 15 14 O O H O O H 50 45 40 35 30 25 20 15 Chemical Shift (ppm) 12.42 18.06 22.39 25.15 29.67 32.28 35.29 41.15 47.72 48.61 48.70 52.17 48.5 48.0 Chemica... 47.7248.61 48.70 200 180 160 140 120 100 80 60 40 20 0 Chemical Shift (ppm) Chloroform -d 18.06 12.42 22.39 25.15 29.67 32.28 41.15 35.29 47.72 48.61 52.17 77.21 77.00 76.79 80.52 177.62 201.40 211.03 177 ppm 211 201
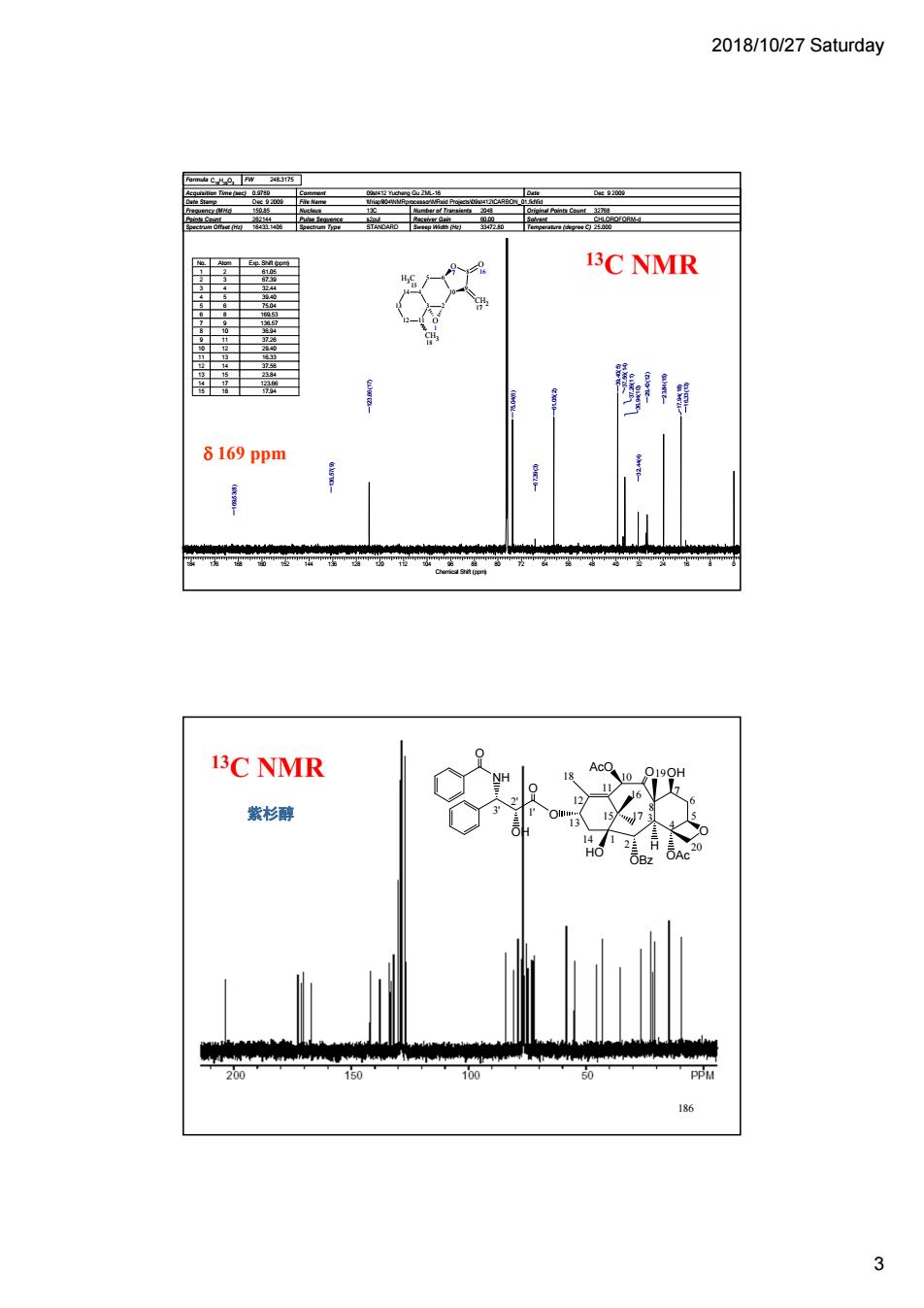
2018/10/27 Saturday 3C NMR 8169 ppm 3C NMR 紫杉醇 3
2018/10/27 Saturday 3 185 Formula C15H20O3 FW 248.3175 Acquisition Time (sec) 0.9789 Comment 09st412 Yucheng Gu ZML-16 Date Dec 9 2009 Date Stamp Dec 9 2009 File Name \\friapfil04\NMRprocessor\MReid Projects\09st412\CARBON_01.fid\fid Frequency (MHz) 150.85 Nucleus 13C Number of Transients 2048 Original Points Count 32768 Points Count 262144 Pulse Sequence s2pul Receiver Gain 60.00 Solvent CHLOROFORM-d Spectrum Offset (Hz) 16433.1406 Spectrum Type STANDARD Sweep Width (Hz) 33472.80 Temperature (degree C) 25.000 6 O 7 10 8 9 5 4 3 2 14 13 12 11 O 16 CH2 17 O 1 CH3 18 H C3 15 184 176 168 160 152 144 136 128 120 112 104 96 88 80 72 64 56 48 40 32 24 16 8 0 Chemical Shift (ppm) 16.33(13) 17.94(18) 29.40(12) 23.84(15) 32.44(4) 36.94(10) 37.26(11) 37.56(14) 39.40(5) 61.05(2) 67.39(3) 75.04(6) 123.66(17) 136.57(9) 169.53(8) No. Atom Exp. Shift (ppm) 1 2 61.05 2 3 67.39 3 4 32.44 4 5 39.40 5 6 75.04 6 8 169.53 7 9 136.57 8 10 36.94 9 11 37.26 10 12 29.40 11 13 16.33 12 14 37.56 13 15 23.84 14 17 123.66 15 18 17.94 169 ppm 186 HO OAc O OBz H O O OH O NH OH O AcO 2 4 7 10 13 15 1' 2' 18 19 20 16 3' 5 6 3 14 1 12 11 17 8 紫杉醇
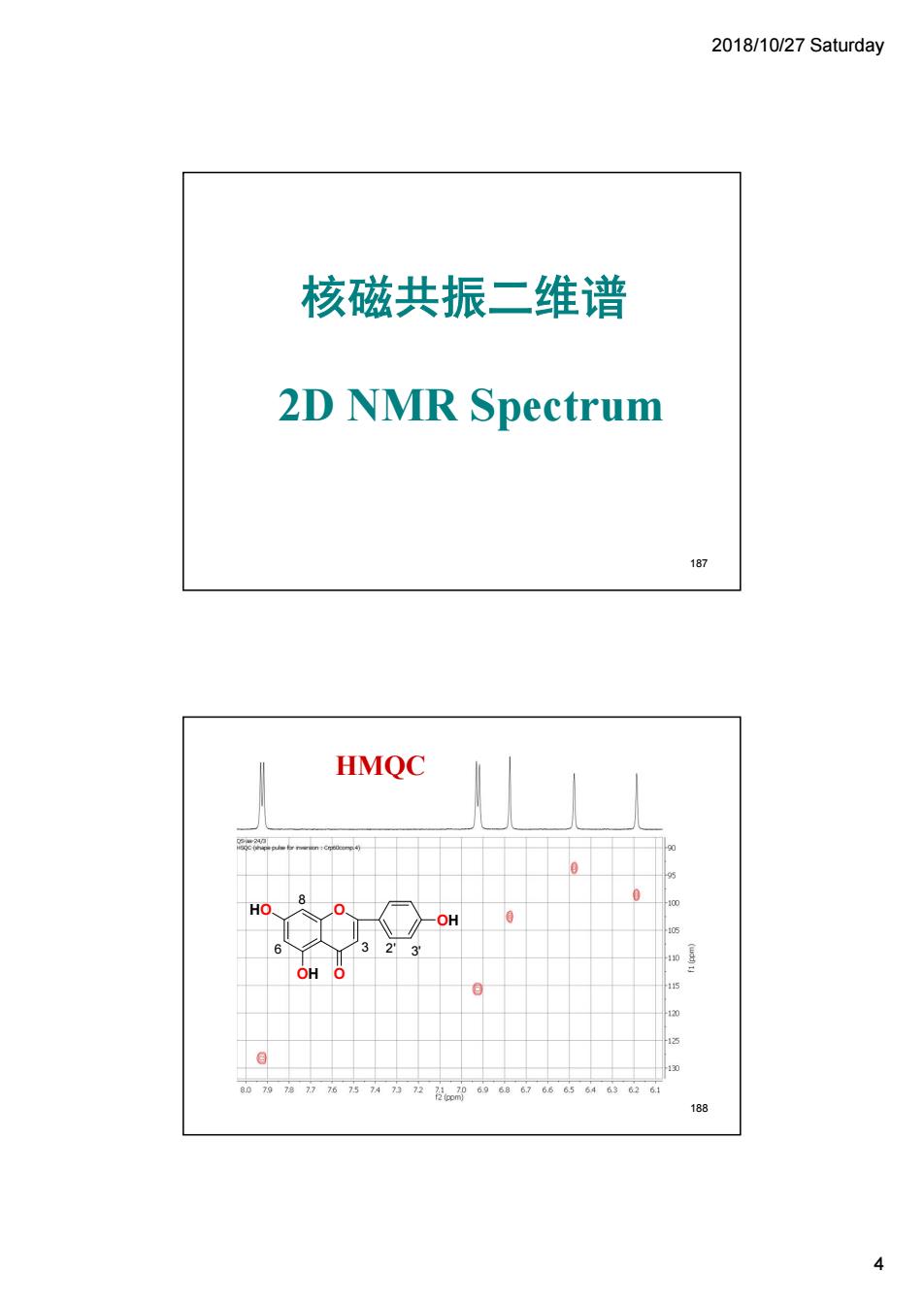
2018/10/27 Saturday 核磁共振二维谱 2D NMR Spectrum 187 HMQC 的9”050050 188
2018/10/27 Saturday 4 核磁共振二维谱 2D NMR Spectrum 187 O O OH OH HO 6 3 8 2' 3' HMQC 188
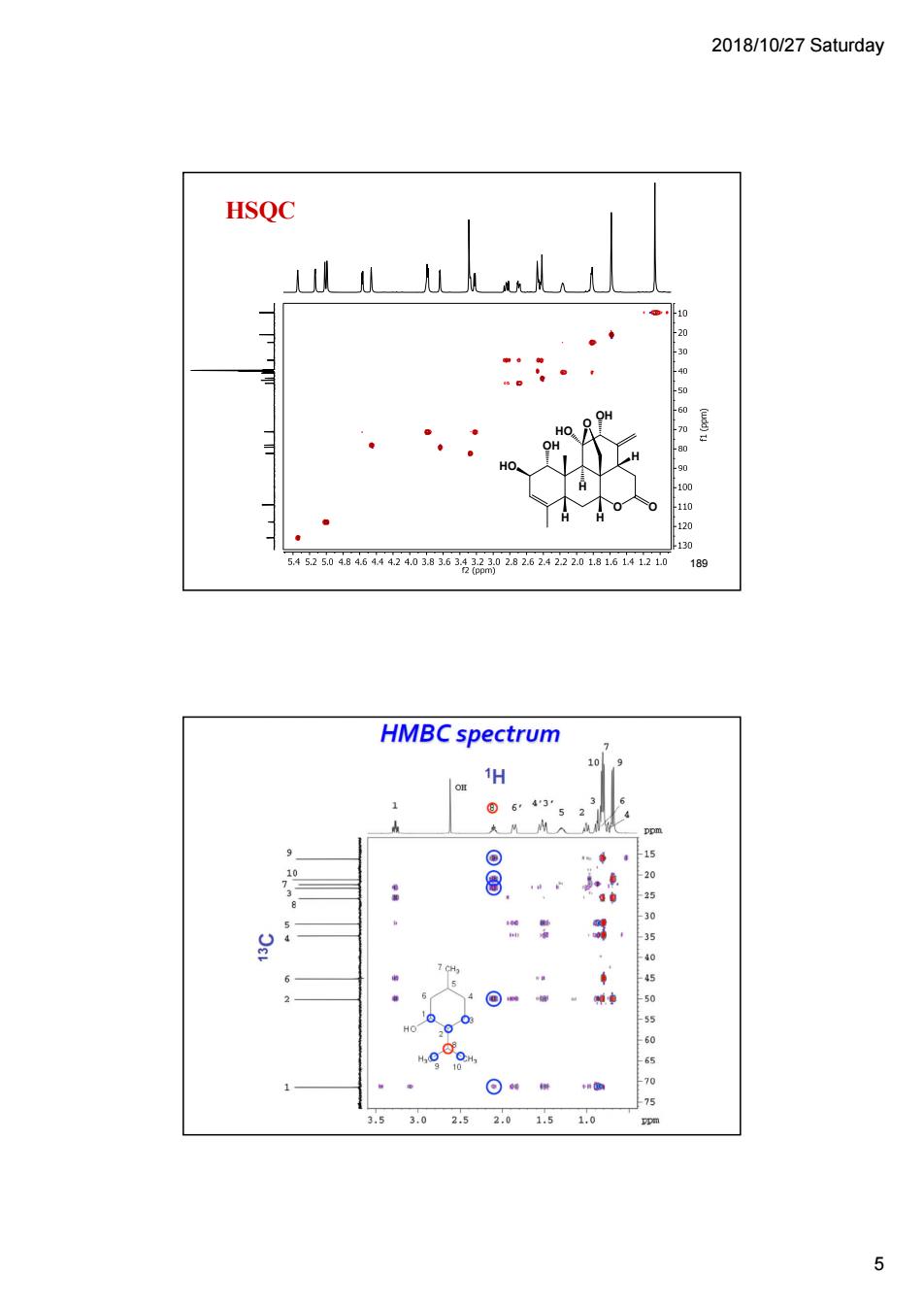
2018/10/27 Saturday HSQC 520464203036g20202624222011614120 189 HMBC spectrum /o 43 5 .5 3025 5
2018/10/27 Saturday 5 HSQC O O HO O OH OH HO H H H H 189 190

2018/10/27 Saturday H3C0、 K-ocH OH OH O 200T180T160T10T 6
2018/10/27 Saturday 6 O O OCH3 OH H3CO OH 191 O O OCH3 OH H3CO OH 192
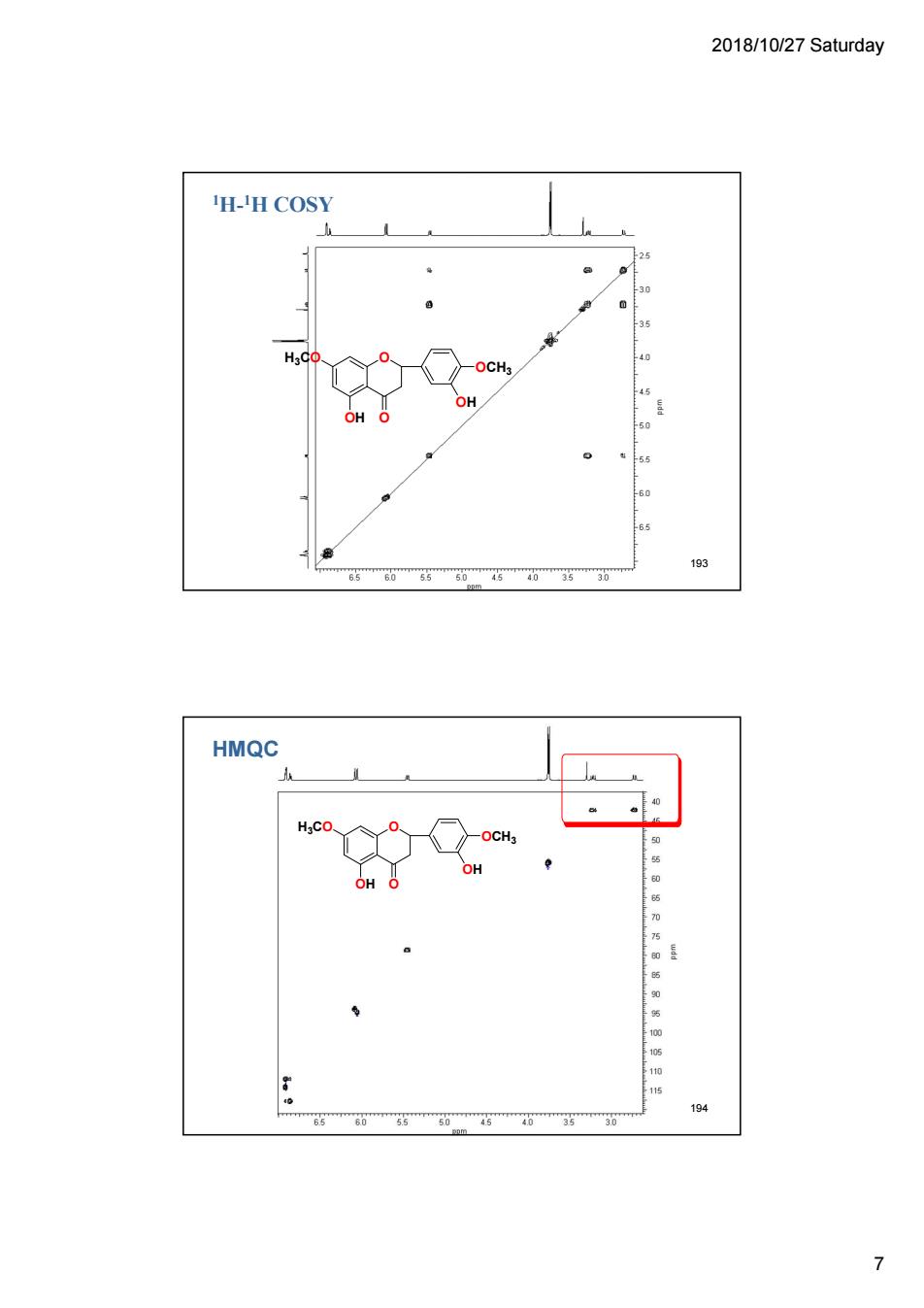
2018/10/27 Saturday H-H COSY H.C 193 60 4036 HMQC OCH: 60 66 7
2018/10/27 Saturday 7 O O OCH3 OH H3CO OH 1H-1H COSY 193 HMQC O O OCH3 OH H3CO OH 194
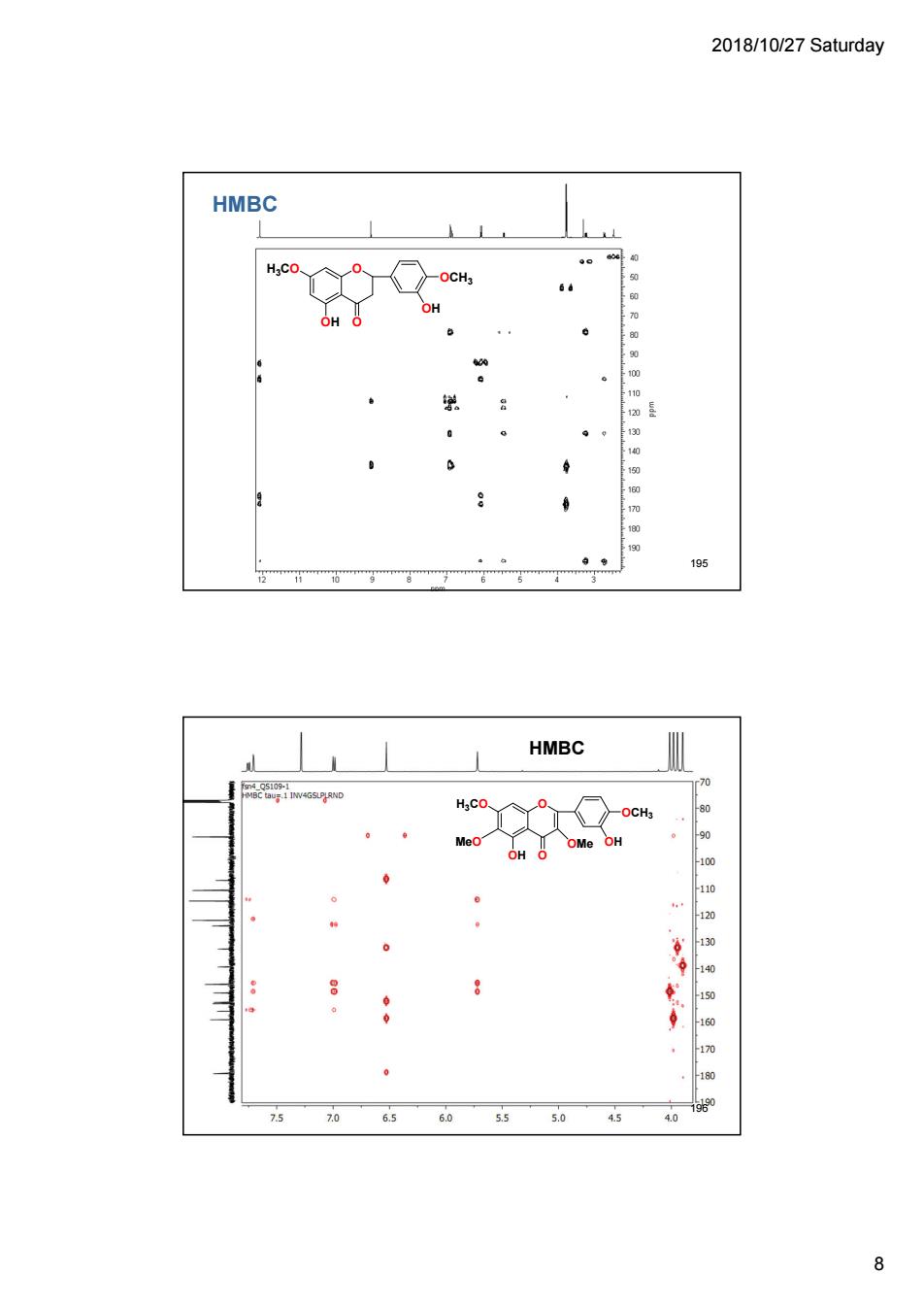
2018/10/27 Saturday HMBC OCH OH 195 HMBC H:C Me 75 60 55 50 45 8
2018/10/27 Saturday 8 HMBC O O OCH3 OH H3CO OH 195 HMBC O O OCH3 OH H3CO MeO OMe OH 196
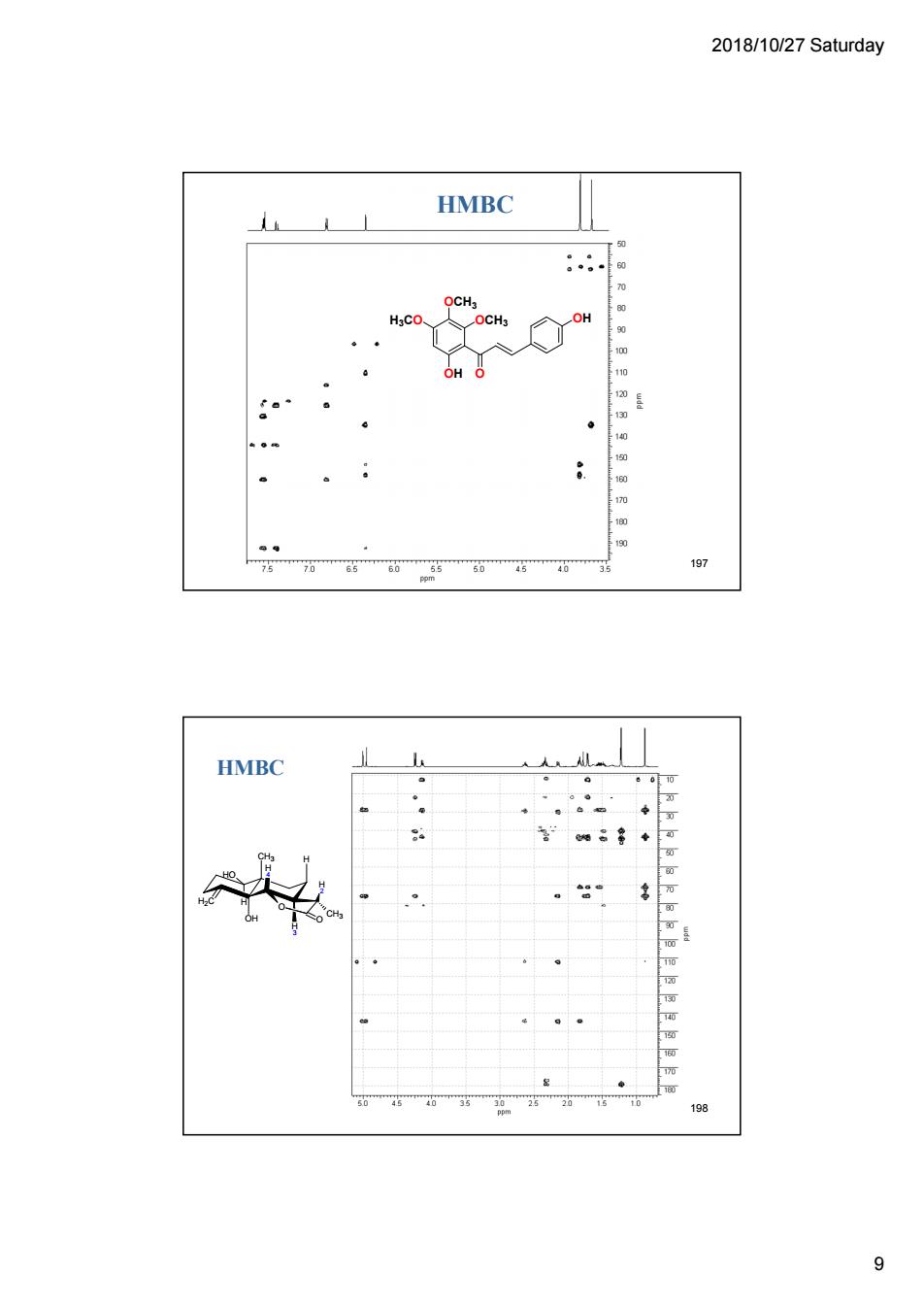
2018/10/27 Saturday HMBC OCH, 197 HMBC 198 9
2018/10/27 Saturday 9 HMBC OCH3 OH O H3CO OCH3 OH 197 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 HMBC 198

2018/10/27 Saturday 波谱法特点 ◆()样品用量少,一般来说2-3mg即可 (最低可少到<1mg); ·(②)除质谱外,其它方法无样品消耗,可回收再 使用; ◆(3)省时、简便 ◆(④)配合元素分析(或高分辨质谱),可以准确 地确定化合物的结构 19g Ciguatoxin CTX 如人·有萧有-含鼠明 1.1 mg from 4000 Kg of moray eels A C55 fatty acid coiled into one terminal spiro and twelve contiguous transfused ether rings ranging in size from oxolane to oxonane.The remaining structural features were unremarkable-five olefins,five methyls,and six hydroxyls,two as a terminal vicinal diol. LD00.254g/kg)200 10
2018/10/27 Saturday 10 波谱法特点 (1) 样品用量少,一般来说2-3 mg即可 (最低可少到<1 mg); (2) 除质谱外,其它方法无样品消耗,可回收再 使用; (3) 省时、简便 (4) 配合元素分析(或高分辨质谱),可以准确 地确定化合物的结构 199 Ciguatoxin CTX A C55 fatty acid coiled into one terminal spiro and twelve contiguous transfused ether rings ranging in size from oxolane to oxonane. The remaining structural features were unremarkable-five olefins, five methyls, and six hydroxyls, two as a terminal vicinal diol. O O O O O O O O O O O O O H H H H H HO H H H H OH H OH H H H H H H H H H H OH HO OH (LD50 0.25-4 μg/kg) 1.1 mg from 4000 Kg of moray eels 200