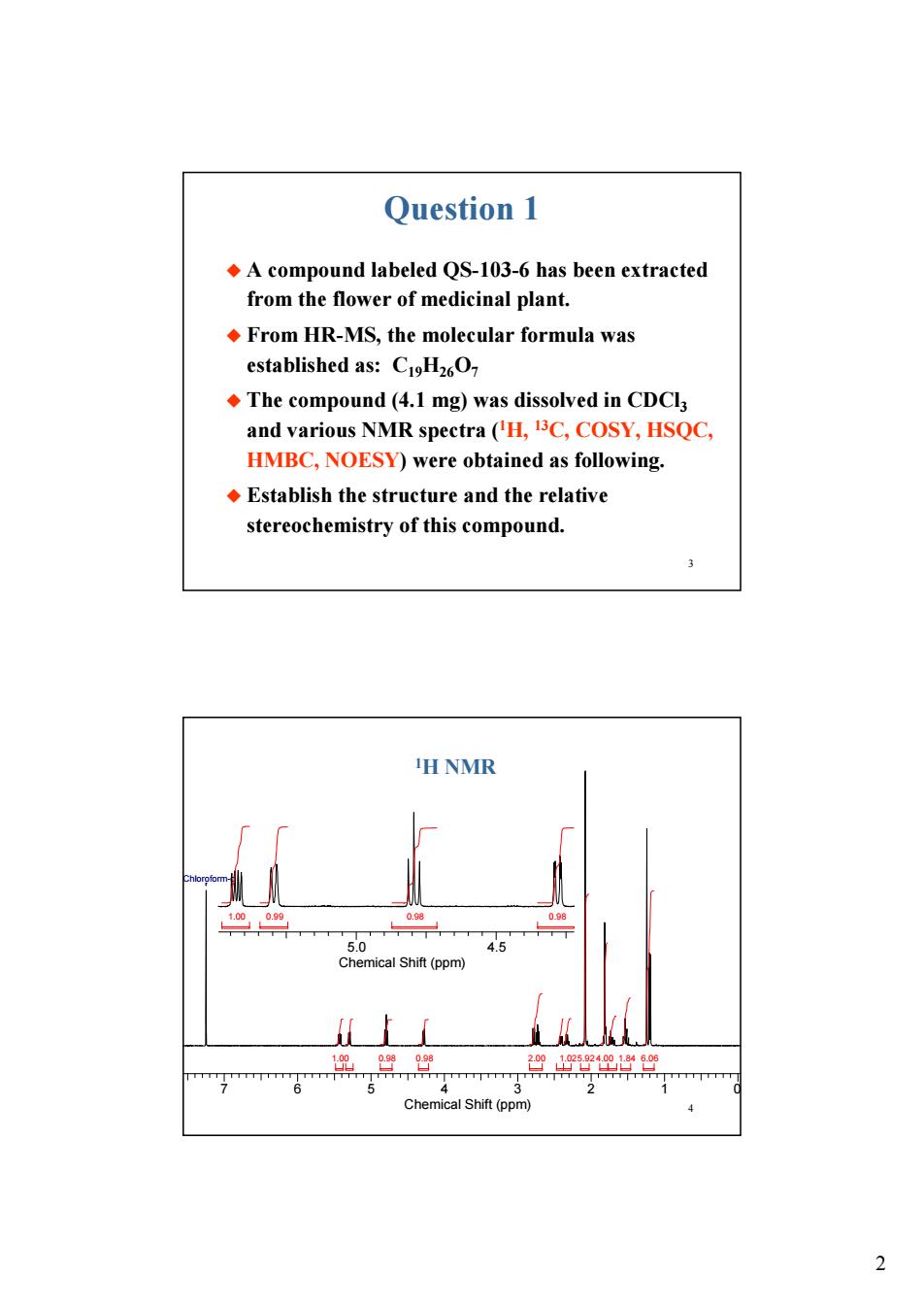
Question 1 A compound labeled QS-103-6 has been extracted from the flower of medicinal plant. From HR-MS,the molecular formula was established as:C1H26O The compound(4.1 mg)was dissolved in CDCla and various NMR speetra (H,13C,COSY,HSQC. HMBC,NOESY)were obtained as following. Establish the structure and the relative stereochemistry of this compound. H NMR 45 ical Shift (ppm) 2
2 3 Question 1 u A compound labeled QS-103-6 has been extracted from the flower of medicinal plant. u From HR-MS, the molecular formula was established as: C19H26O7 u The compound (4.1 mg) was dissolved in CDCl3 and various NMR spectra (1H, 13C, COSY, HSQC, HMBC, NOESY) were obtained as following. u Establish the structure and the relative stereochemistry of this compound. 4 7 6 5 4 3 2 1 0 Chemical Shift (ppm) 1.00 0.98 0.98 2.00 1.025.92 4.00 1.84 6.06 Chloroform-d 5.0 4.5 Chemical Shift (ppm) 1.00 0.99 0.98 0.98 1H NMR
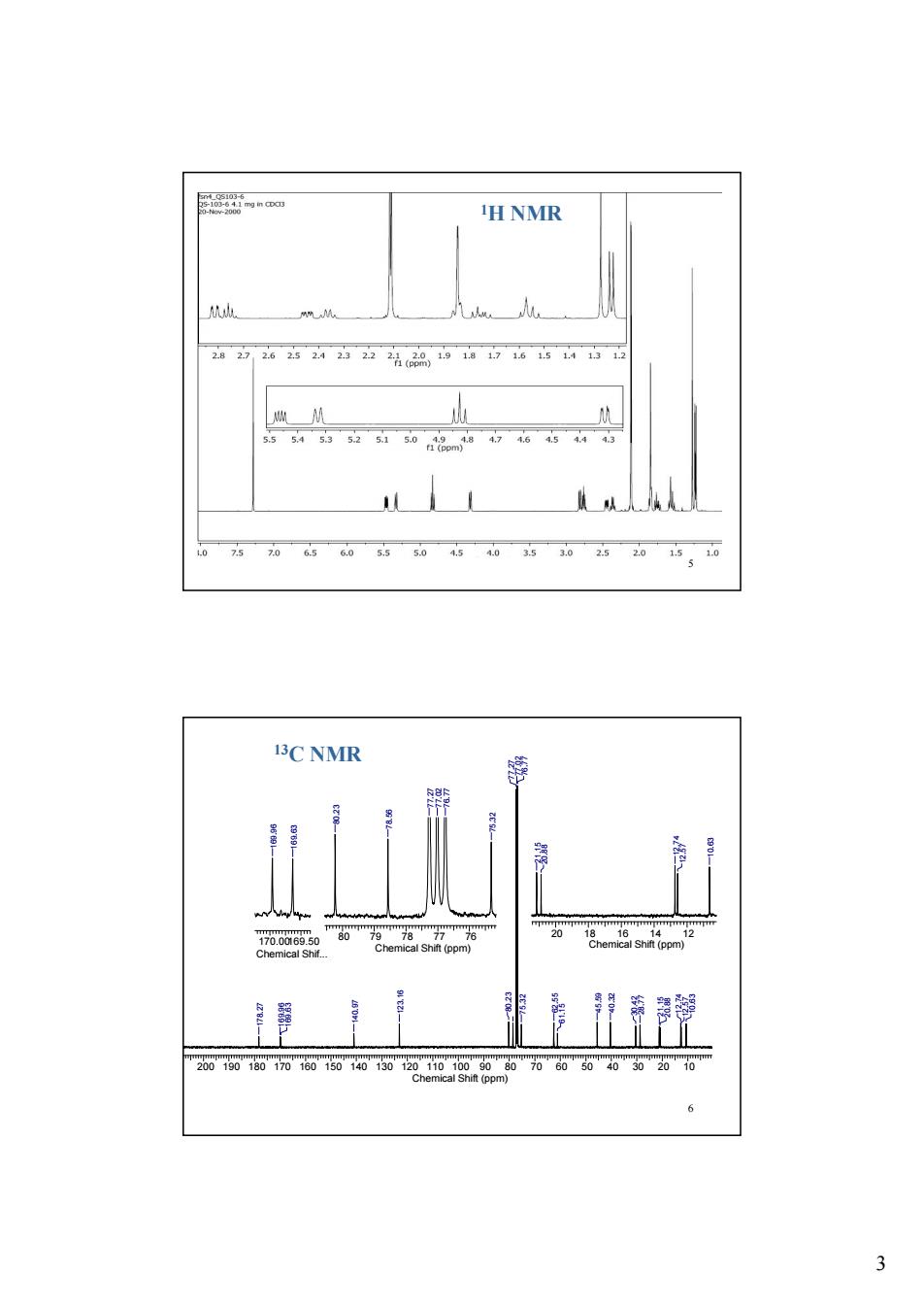
H NMR 282262524232222品19116154112 1 5.5 C NMR 力gem8spm2 20的00050w0200000的020动260 3
3 5 1H NMR 6 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 Chemical Shift (ppm) 178.27 169.96 169.63 140.97 123.16 80.23 77.27 77.02 76.77 75.32 62.55 61.15 45.59 40.32 30.42 28.77 21.15 20.88 12.74 12.57 10.63 170.00169.50 Chemical Shif... 169.96 169.63 80 79 78 77 76 Chemical Shift (ppm) 80.23 78.56 77.27 77.02 76.77 75.32 20 18 16 14 12 Chemical Shift (ppm) 21.15 20.88 12.74 12.57 10.63 13C NMR
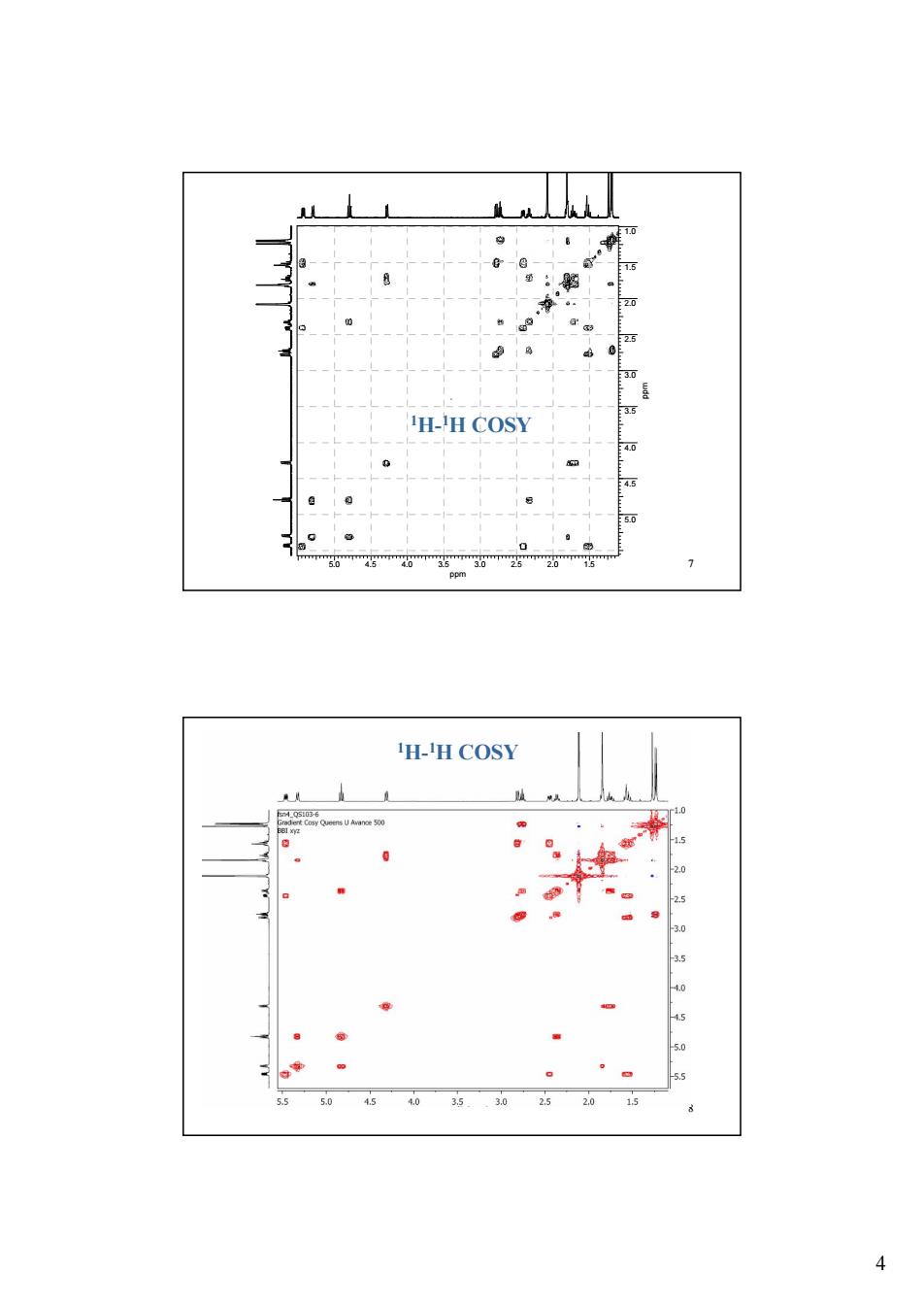
H-H COSY 西 。 H-H COSY · 5 25 45 55 505035302620
4 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 7 ppm 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 ppm 1H-1H COSY 8 1H-1H COSY

o 3030462983030262218w HMBC 50 6303302622181 5
5 9 HSQC 10 HMBC
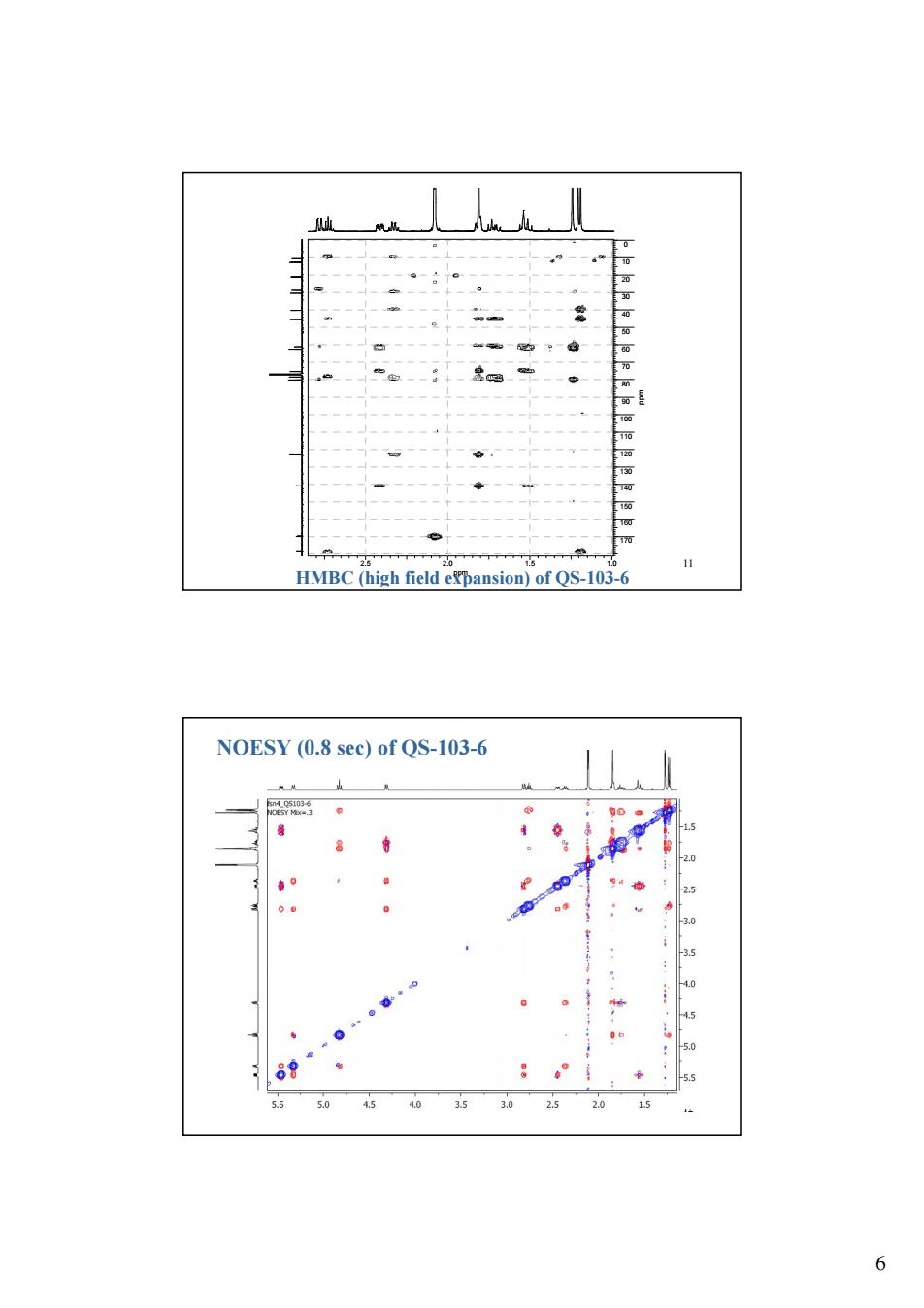
11 HMBC (high field expansion)of QS-103-6 NOESY (0.8 sec)of QS-103-6 3.0 3.5 80 5.5 45 30 6
6 11 HMBC (high field expansion) of QS-103-6 2.5 2.0 1.5 1.0 ppm 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 ppm 12 NOESY (0.8 sec) of QS-103-6

Structure??? What information we have gotten from each spectrum? How to use these information to establish a structure? What Is Structure Elucidation? From a chemist's standpoint,a structure is a three dimensional array of atoms and solving a structure is not only finding where chemical bonds are but also how they are spatially arranged in the relative as well as in the absolute mode.Finally,molecules have mobility and therefore conformation around bonds and in rings must be defined also. 14
7 13 What information we have gotten What information we have gotten from each spectrum? from each spectrum? How to use these information to How to use these information to establish a structure? establish a structure? Structure??? Structure??? 14 What Is Structure Elucidation? What Is Structure Elucidation? From a chemist’s standpoint, a structure is a three dimensional array of atoms and solving a structure is not only finding where chemical bonds are but also how they are spatially arranged in the relative as well as in the absolute mode. Finally, molecules have mobility and therefore conformation around bonds and in rings must be defined also

What are the tools which permit solving chemical structure? ◆Elemental analysis ◆Mass spectrometry Ultra Violt,Infra Red,Raman,Circular Dichroism Nuclear Magnetic Resonance:proton, carbon-13 ◆X-ray diffraction Chemistry Structure Elucidation During all of the 19th century and most of the early half of the 20th century,natural produet structure elucidation was an art that depended almost entirely on the power of chemical synthesis,or,more specifically,on the effectiveness of degradation or derivatization processes,to reveal the architectural design of a molecule.Assuming both that gram quantities of the substance under investigation were available and that the chemical transformations employed proceeded along expected lines,researchers of that era might have expected to solve their molecular puzzles after a few years of painstaking effort. 16 8
8 15 What are the tools which permit What are the tools which permit solving chemical structure? solving chemical structure? u Elemental analysis u Mass spectrometry u Ultra Violt, Infra Red, Raman, Circular Dichroism u Nuclear Magnetic Resonance: proton, carbon-13 u X-ray diffraction u Chemistry 16 Structure Elucidation Structure Elucidation During all of the 19th century and most of the early half of the 20th century, natural product structure elucidation was an art that depended almost entirely on the power of chemical synthesis, or, more specifically, on the effectiveness of degradation or derivatization processes, to reveal the architectural design of a molecule. Assuming both that gram quantities of the substance under investigation were available and that the chemical transformations employed proceeded along expected lines, researchers of that era might have expected to solve their molecular puzzles after a few years of painstaking effort

Structure Elucidation During all of the 19th century and most of the early half of the 20th century,the assignment of absolute or relative configuration was,of course, essentially out of the question in most cases. Needless to say,this intellectually difficult and physically tedious approach had its limitations,and was often attended with errors. Examples of Structure Elucidation 118 Years 952 Total Syn By Gat 高怡生 Syn.In 1954 广方 9
9 17 Structure Elucidation Structure Elucidation During all of the 19th century and most of the early half of the 20th century, the assignment of absolute or relative configuration was, of course, essentially out of the question in most cases. Needless to say, this intellectually difficult and physically tedious approach had its limitations, and was often attended with errors. 18 Examples of Structure Elucidation O HO NCH3 H HO H N O H H H H O H N Morphine 118 Years! Strychnine 127 Years! Robert Robinson published 54 paper for structure elucidation And determined it in 1946 and got Nobel Prize in 1947. Robert B. Woodward Total Syn. In 1954. Robert Robinson 1925 (1886-1975) 1952 Total Syn By Gates 高怡生 曾广方
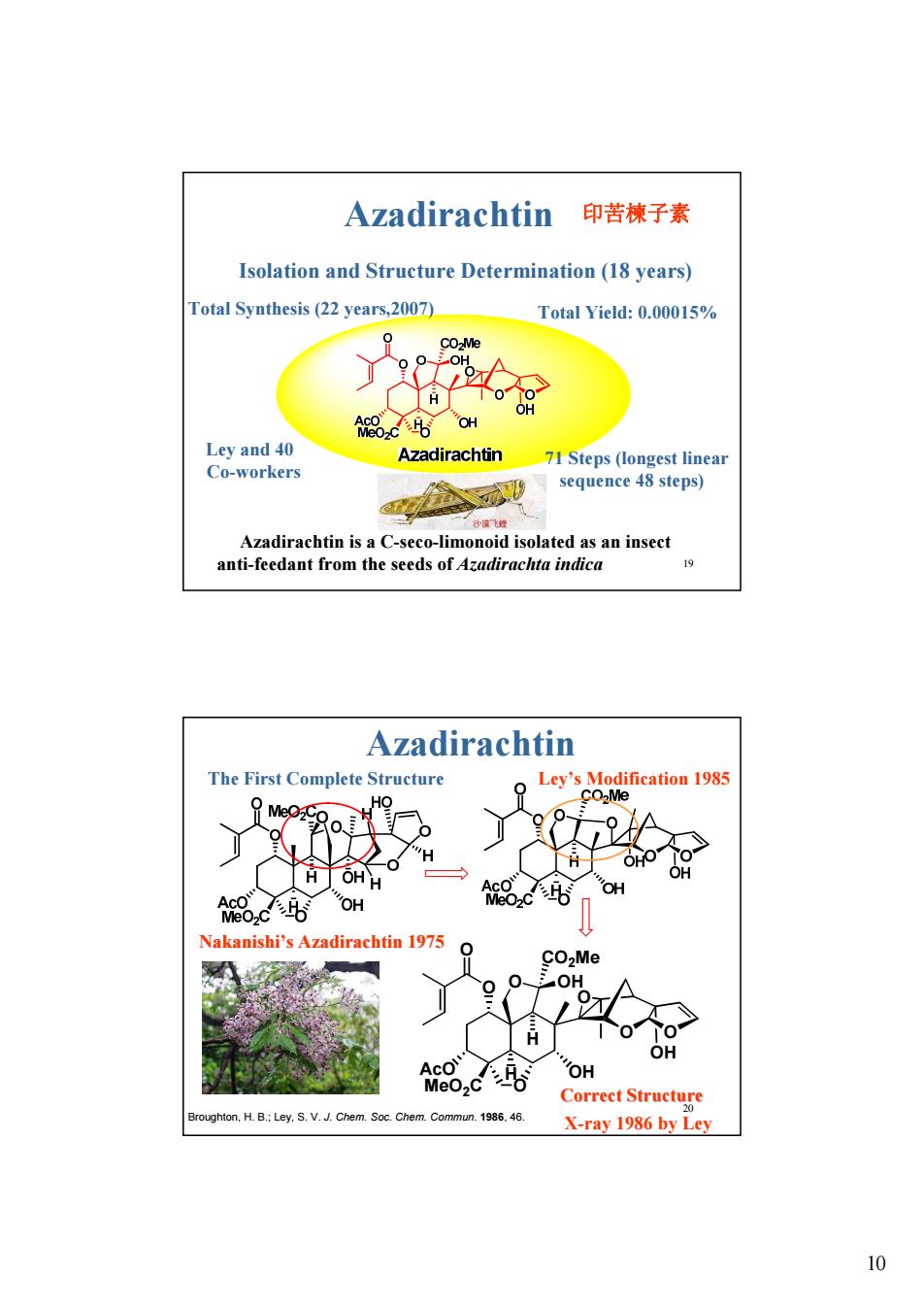
Azadirachtin印苦楝子素 Isolation and Structure Determination(18 years) Total Synthesis(22 years,2007) Total Yield:0.00015% 8Cor Ley and 40 Azadirachtin 71 Steps (longest linea Co-workers sequence 48 steps) Azadirachtin is a C-seco-limonoid isolated as an insect anti-feedant from the seeds of Azadirachta indica Azadirachtin The First Complete Structure Ley's Modification 1985 H H OH OH Nakanishi's Azadirachtin 1975 O COMe 。。 OH Ai品,c6 OH Correet Structure ughton,H.B.;Ley.S.V.J.Ch Chem.Commun.1986.46. X-ray 1986 by Ley 10
10 19 Azadirachtin Ley and 40 Co-workers Isolation and Structure Determination (18 years) Total Synthesis (22 years,2007) Total Yield: 0.00015% 71 Steps (longest linear sequence 48 steps) Azadirachtin is a C-seco-limonoid isolated as an insect anti-feedant from the seeds of Azadirachta indica 印苦楝子素 20 The First Complete Structure Ley’s Modification 1985 MeO2C O O O O OH O AcO H O O H OH CO2Me OH Correct Structure Correct Structure Azadirachtin Broughton, H. B.; Ley, S. V. J. Chem. Soc. Chem. Commun. 1986, 46. Nakanishi Nakanishi’s Azadirachtin 1975 X-ray 1986 by Ley

Prof.Nakanishi Koji Koji Nakanghiwas bom in 1925 in Hong years with LF.Fieser at Harvard,he eceived his PhD in 1954 from Nagoya moved to Columbia niversity,where he is currently Centennial Professor of chemistry. rmining the structure of the Comprehensive Natural Products Chemistry 1998 Azadirachtin sees first total synthesis 10 August 2007 A complex natural product has finally succumbed to its first total synthesis after 22 years of attempts by eminent organic chemists. 22 11
11 21 Prof. Nakanishi Koji Comprehensive Natural Products Chemistry 1998 22 Azadirachtin sees first total synthesis 10 August 2007 A complex natural product has finally succumbed to its first total synthesis after 22 years of attempts by eminent organic chemists