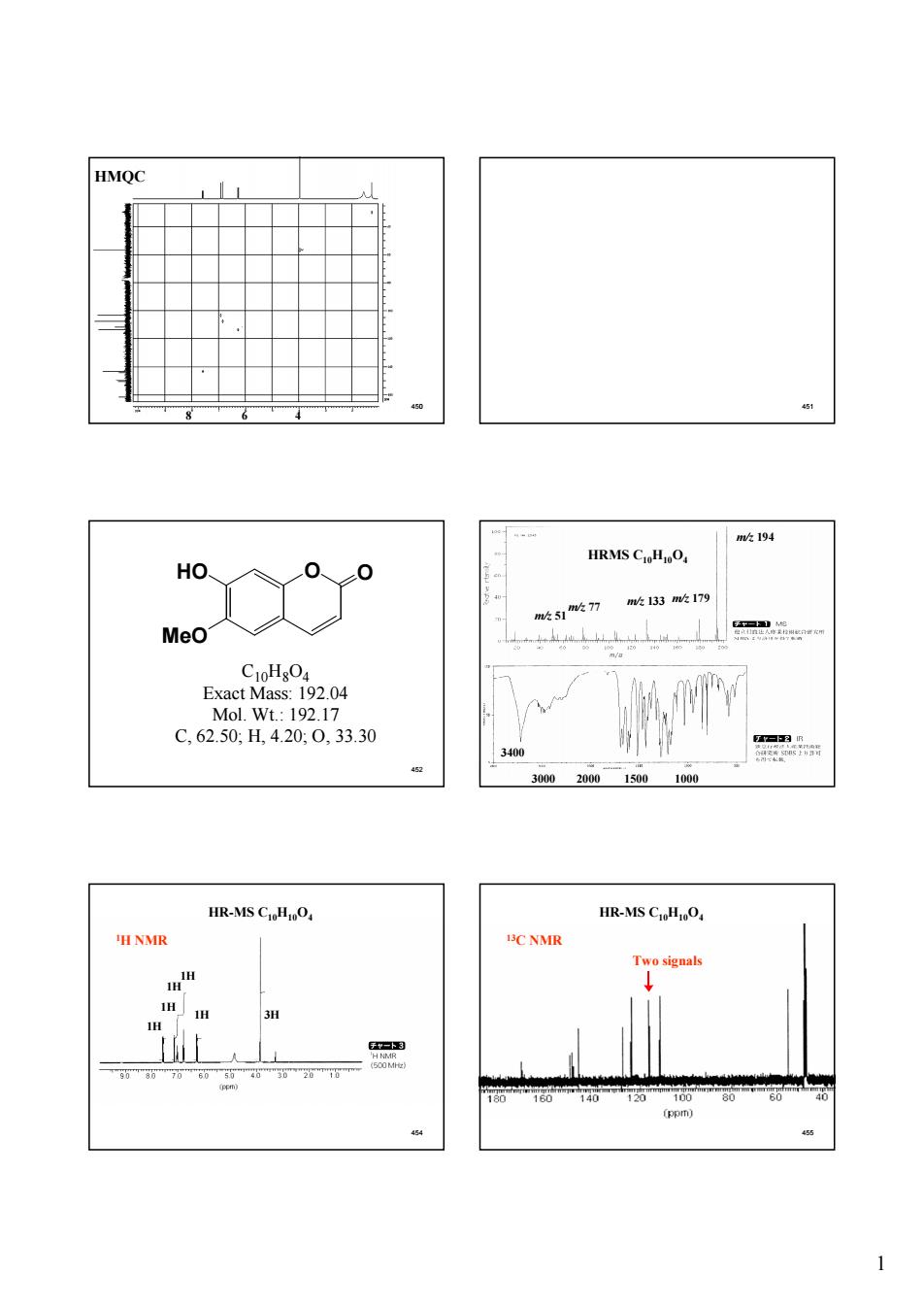
mk194 HRMS C.H,O Ho、 133m吹179 Meo .192.04 Mol.Wt.:192.17 C,62.50H4.20:0.33.30 340 300020001500100 HR-MS CHnO HR-MS CioHjO, C NMR
1 450 HMQC 8 6 4 451 452 O O MeO HO C10H8O4 Exact Mass: 192.04 Mol. Wt.: 192.17 C, 62.50; H, 4.20; O, 33.30 453 m/z 194 m/z 133 m/z 179 m/z 51m/z 77 3400 3000 2000 1500 1000 HRMS C10H10O4 454 HR-MS C10H10O4 1H 1H 1H 1H 1H 3H 1H NMR 455 13C NMR Two signals HR-MS C10H10O4
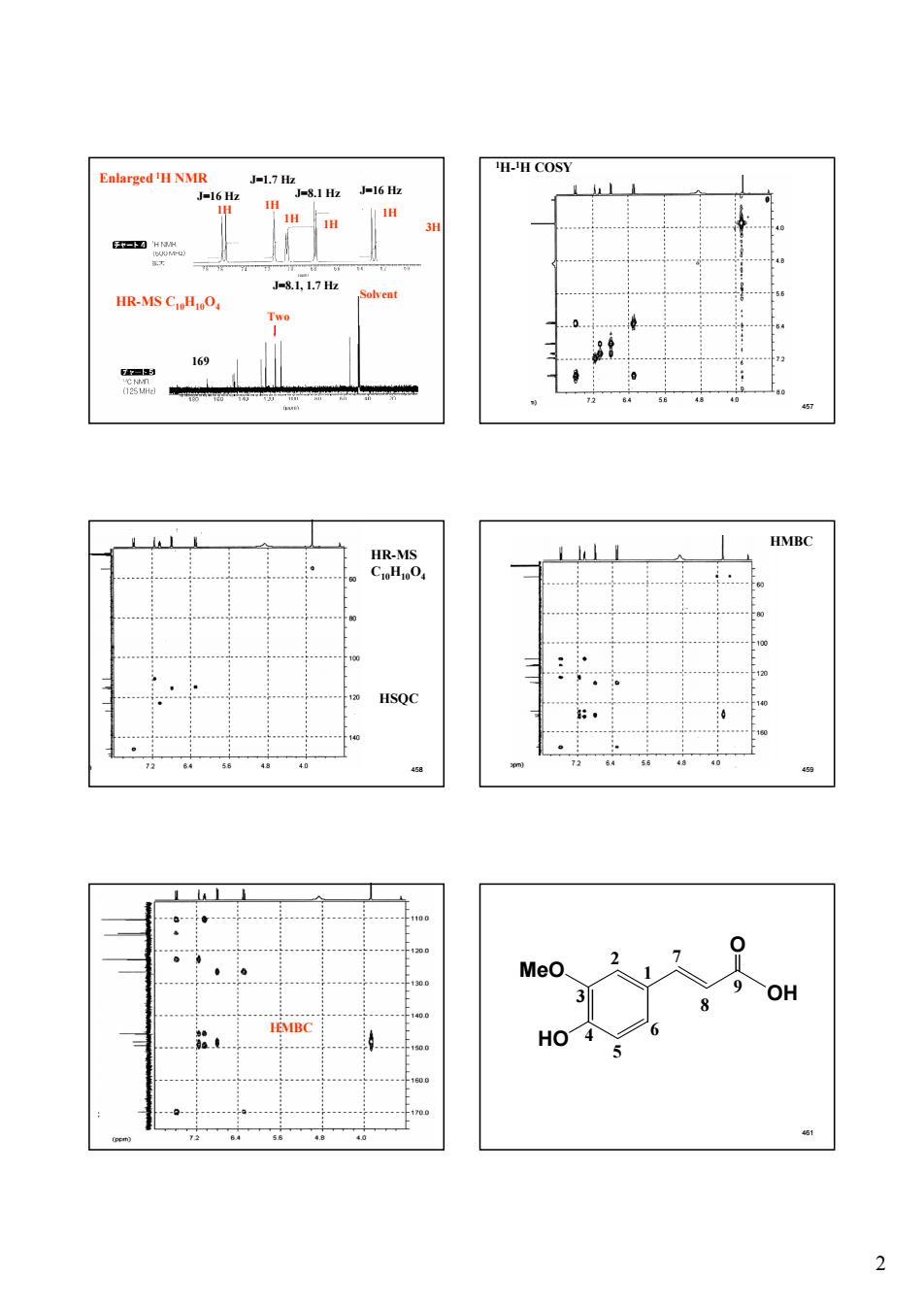
Enlarged用NMR H-H COSY J17 62 81z6 HR-MS Two 169 HMB OH
2 456 169 J=16 Hz J=16 Hz J=1.7 Hz J=8.1 Hz J=8.1, 1.7 Hz HR-MS C10H10O4 3H 1H 1H 1H 1H 1H Solvent Two Enlarged 1H NMR 457 1H-1H COSY 458 HR-MS C10H10O4 HSQC 459 HMBC 460 HMBC 461 OH O HO MeO 1 2 3 4 5 7 6 8 9
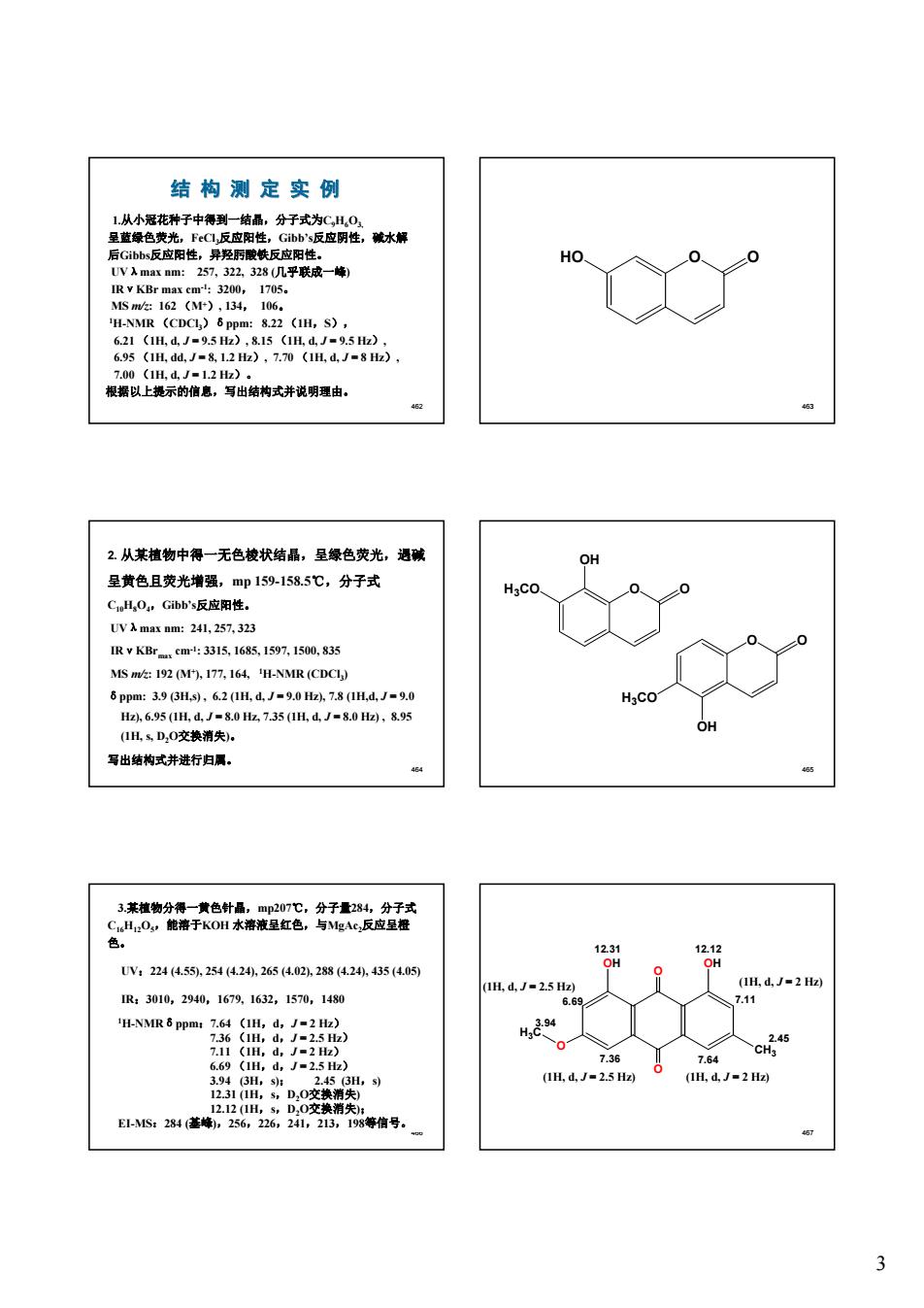
结构测定实例 HO. H-NMIR ( 822(1H,s, 根以上境示的信意,写出结构式并说明理由。 2从某植物中得一无色棱状结晶,呈绿色荧光,海碱 呈黄色且荧光增强,mp159-1585℃,分子式 CL.O,Gbhs反应用 g24,257,32 315168,1597.150,835 ().7.4 NMR (CDCL H.co 1H飞D.0交热消奥 写出结构式并进行归属。 来毒如8:种 UV22445.25420,265(a02.282,435(05 H,d.J=2.5 Hz 1H.4.J=2H IR:3010,2940,1679.1632,1570,1480 764 (IH.d,J-2.5 H) (IH,d.J-2H)
3 462 结 构 测 定 实 例 1.从小冠花种子中得到一结晶,分子式为C9H6O3, 呈蓝绿色荧光,FeCl3反应阳性,Gibb’s反应阴性,碱水解 后Gibbs反应阳性,异羟肟酸铁反应阳性。 UVλmax nm: 257, 322, 328 (几乎联成一峰) IRνKBr max cm-1: 3200, 1705。 MS m/z: 162 (M+), 134, 106。 1H-NMR (CDCl3)δppm: 8.22 (1H,S), 6.21 (1H, d, J = 9.5 Hz), 8.15 (1H, d, J = 9.5 Hz), 6.95 (1H, dd, J = 8, 1.2 Hz), 7.70 (1H, d, J = 8 Hz), 7.00 (1H, d, J = 1.2 Hz)。 根据以上提示的信息,写出结构式并说明理由。 463 HO O O 464 2. 从某植物中得一无色棱状结晶,呈绿色荧光,遇碱 呈黄色且荧光增强,mp 159-158.5℃,分子式 C10H8O4,Gibb’s反应阳性。 UVλmax nm: 241, 257, 323 IRνKBrmax cm-1: 3315, 1685, 1597, 1500, 835 MS m/z: 192 (M+), 177, 164, 1H-NMR (CDCl3) δppm: 3.9 (3H,s) , 6.2 (1H, d, J = 9.0 Hz), 7.8 (1H,d, J = 9.0 Hz), 6.95 (1H, d, J = 8.0 Hz, 7.35 (1H, d, J = 8.0 Hz) , 8.95 (1H, s, D2O交换消失)。 写出结构式并进行归属。 465 O O OH H3CO O O OH H3CO 466 3.某植物分得一黄色针晶,mp207℃,分子量284,分子式 C16H12O5,能溶于KOH 水溶液呈红色,与MgAc2反应呈橙 色。 UV:224 (4.55), 254 (4.24), 265 (4.02), 288 (4.24), 435 (4.05) IR:3010,2940,1679, 1632,1570,1480 1H-NMRδppm:7.64 (1H,d,J = 2 Hz) 7.36 (1H,d,J = 2.5 Hz) 7.11 (1H,d,J = 2 Hz) 6.69 (1H,d,J = 2.5 Hz) 3.94 (3H,s); 2.45 (3H,s) 12.31 (1H,s,D2O交换消失) 12.12 (1H,s,D2O交换消失); EI-MS:284 (基峰),256,226,241,213,198等信号。 467 O O OH OH CH3 O H3C 6.69 7.36 7.64 7.11 12.31 12.12 2.45 3.94 (1H, d, J = 2.5 Hz) (1H, d, J = 2 Hz) (1H, d, J = 2 Hz) (1H, d, J = 2.5 Hz)
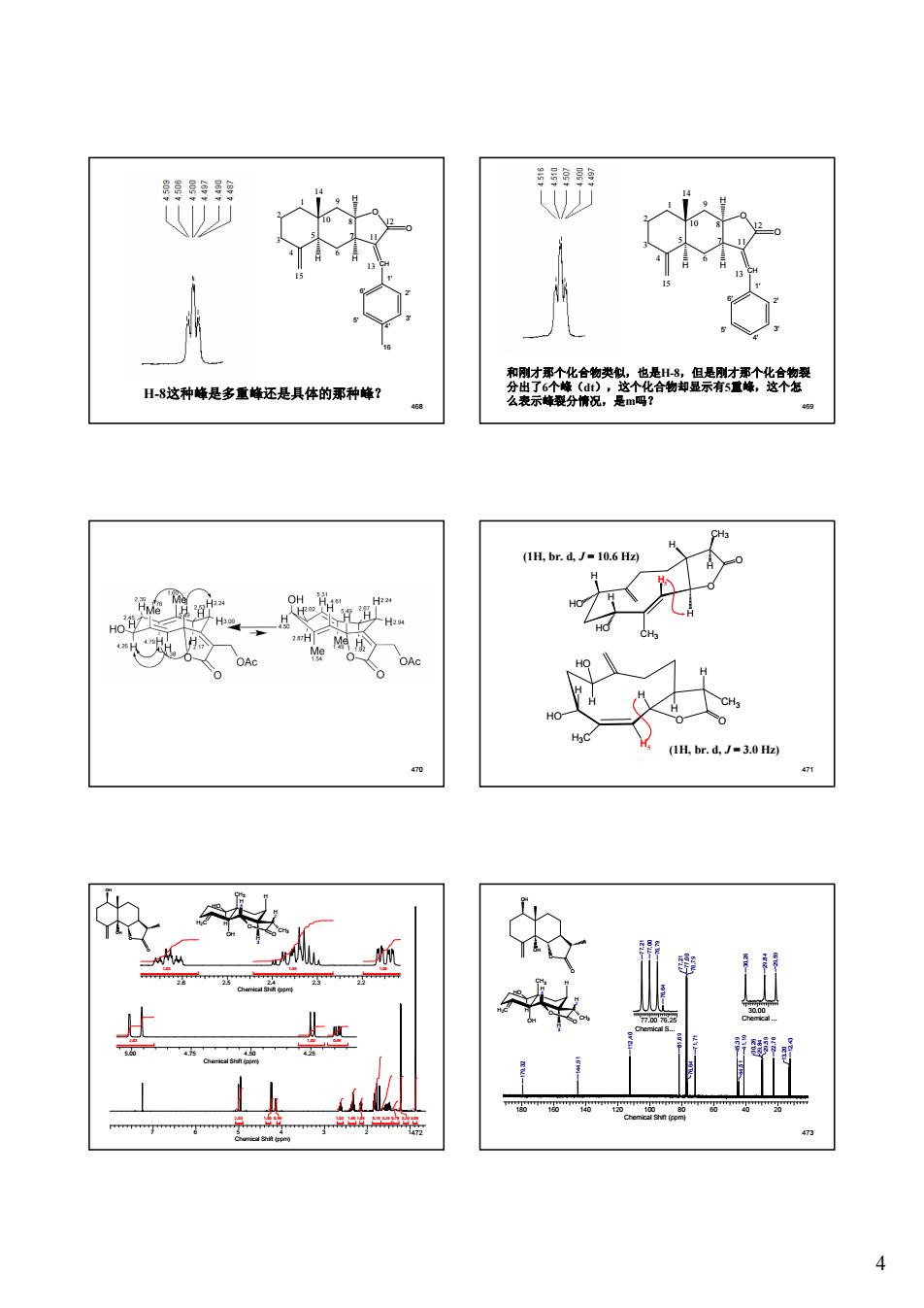
閉 H8这种峰是多重峰还是具体的那种峰 安微 44 IH.br.d.J-3.0 Hz) rt 业
4 468 O CH H H H O 1 2 3 4 5 6 7 8 9 10 11 12 14 15 13 1' 2' 3' 4' 5' 6' 16 H-8这种峰是多重峰还是具体的那种峰? 469 O CH H H H O 1 2 3 4 5 6 7 8 9 10 11 12 14 15 13 1' 2' 3' 4' 5' 6' 和刚才那个化合物类似,也是H-8,但是刚才那个化合物裂 分出了6个峰(dt),这个化合物却显示有5重峰,这个怎 么表示峰裂分情况,是m吗? 470 471 (1H, br. d, J = 10.6 Hz) (1H, br. d, J = 3.0 Hz) O CH3 O H H CH3 H H HO HO H H5 O CH3 O H H H3C H H HO HO H H5 7 6 5 4 3 2 1472 Chemical Shift (ppm) 2.03 1.00 0.99 1.03 1.96 1.05 5.15 3.26 0.78 3.20 3.05 5.00 4.75 4.50 4.25 Chemical Shift (ppm) 2.03 1.00 0.99 2.6 2.5 2.4 2.3 2.2 Chemical Shift (ppm) 1.03 1.96 1.05 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 O OH OH O 473 O OH OH O 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) 12.43 13.20 29.59 22.76 30.26 29.84 41.19 44.51 45.39 71.71 76.64 76.79 77.21 77.00 81.69 112.40 144.91 179.32 77.00 76.25 Chemical S... 76.64 77.21 77.00 76.79 30.00 Chemical ...29.59 30.26 29.84 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3
HMOC 监风 H-NMR
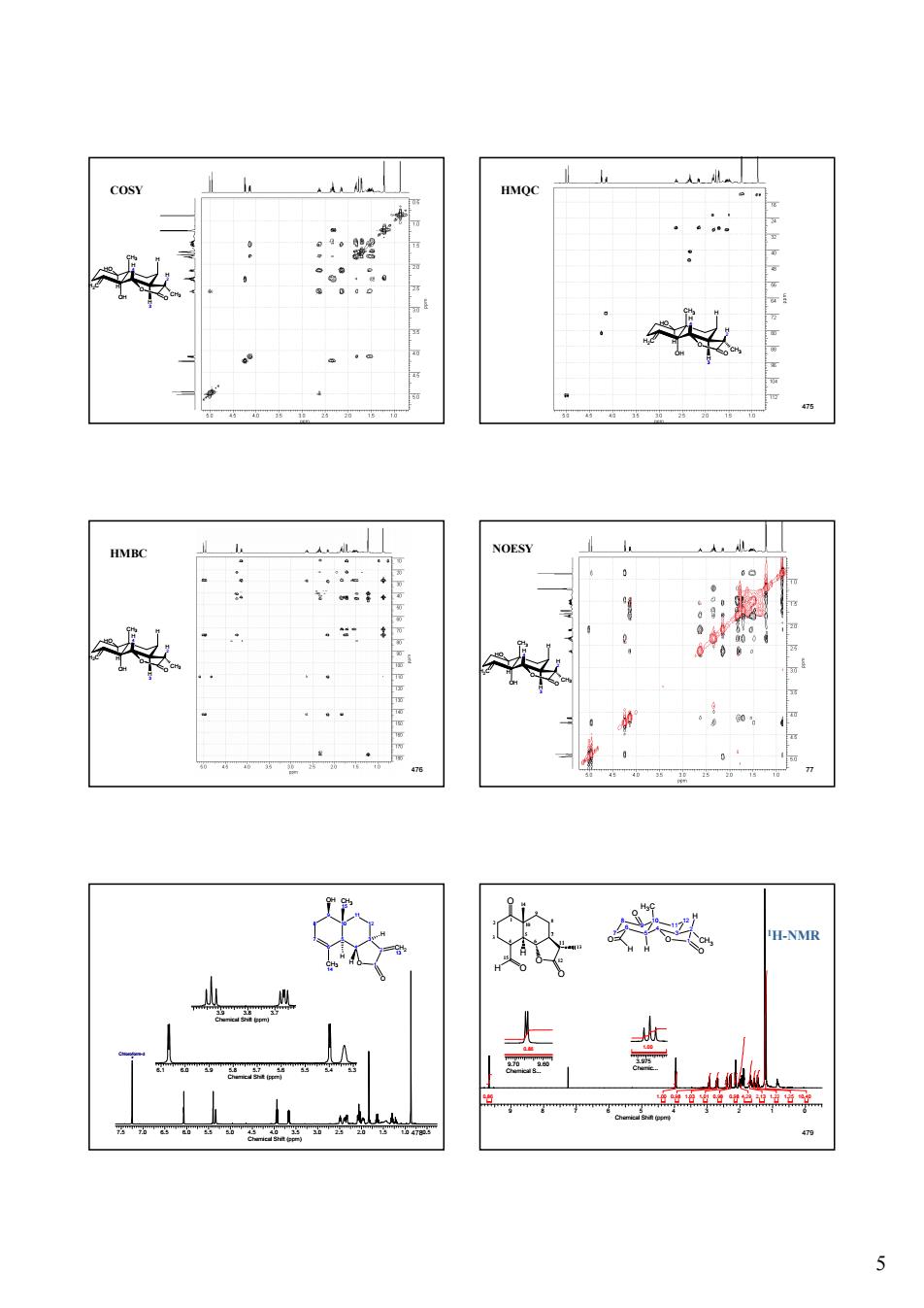
5 474 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 COSY 475 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 HMQC 476 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 HMBC 477 H2C CH3 OH H O O H 4 H 3 H HO H 2 CH3 NOESY 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 4780.5 Chemical Shift (ppm) Chloroform-d 6.1 6.0 5.9 5.8 5.7 5.6 5.5 5.4 5.3 Chemical Shift (ppm) 3.9 3.8 3.7 Chemical Shift (ppm) 10 5 9 6 8 7 12 3 11 4 2 O 1 CH3 15 OH CH3 14 H H H CH2 13 O 479 1 2 3 4 5 6 7 10 9 8 11 12 13 15 14 O O H O O H 7 8 9 10 5 6 11 12 3 4 H3C H O H O O 2 1 O CH3 H 9 8 7 6 5 4 3 2 1 0 Chemical Shift (ppm) 0.86 1.00 0.98 1.03 1.01 0.98 0.98 4.29 2.13 1.22 1.25 10.40 9.70 9.60 Chemical S... 0.86 3.975 Chemic... 1.00 1H-NMR
H-NMR BC-NMR 费 HMQC NOESY . 6
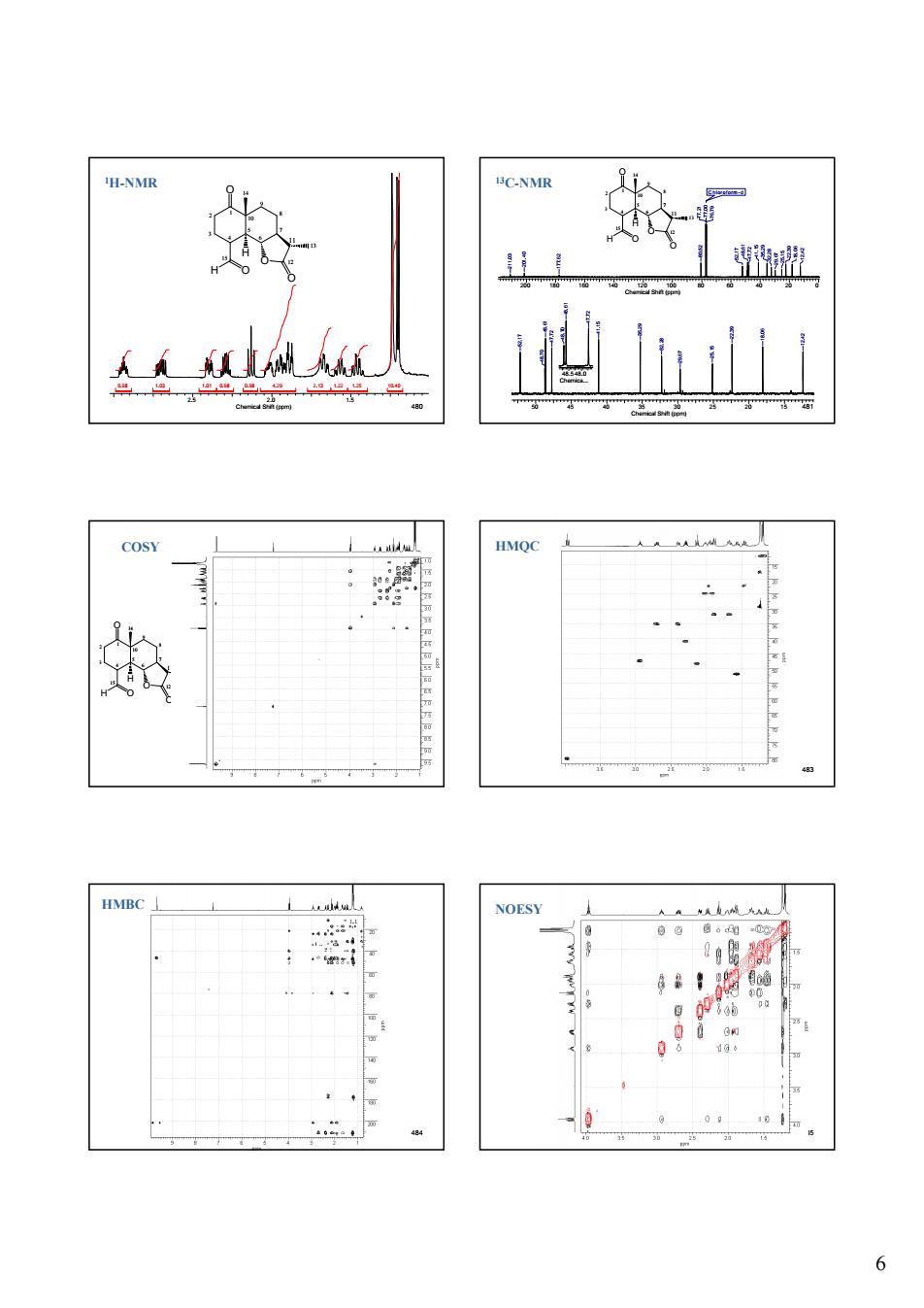
6 480 2.5 2.0 1.5 Chemical Shift (ppm) 0.98 1.03 1.01 0.98 0.98 4.29 2.13 1.22 1.25 10.40 1 2 3 4 5 6 7 10 9 8 11 12 13 15 14 O O H O O H 1H-NMR 481 1 2 3 4 5 6 7 10 9 8 11 12 13 15 14 O O H O O H 50 45 40 35 30 25 20 15 Chemical Shift (ppm) 12.42 18.06 22.39 25.15 29.67 32.28 35.29 41.15 47.72 48.61 48.70 52.17 48.5 48.0 Chemica... 47.7248.61 48.70 200 180 160 140 120 100 80 60 40 20 0 Chemical Shift (ppm) Chloroform -d 18.06 12.42 22.39 25.15 29.67 32.28 41.15 35.29 47.72 48.61 52.17 77.21 77.00 76.79 80.52 177.62 201.40 211.03 13C-NMR 482 1 2 3 4 5 6 7 10 9 8 11 12 13 15 14 O O H O O H COSY 483 HMQC 484 HMBC 485 NOESY
H-NMR H-NMR I-NMR H-NMR H in acelone at lemp-26 HMOC >
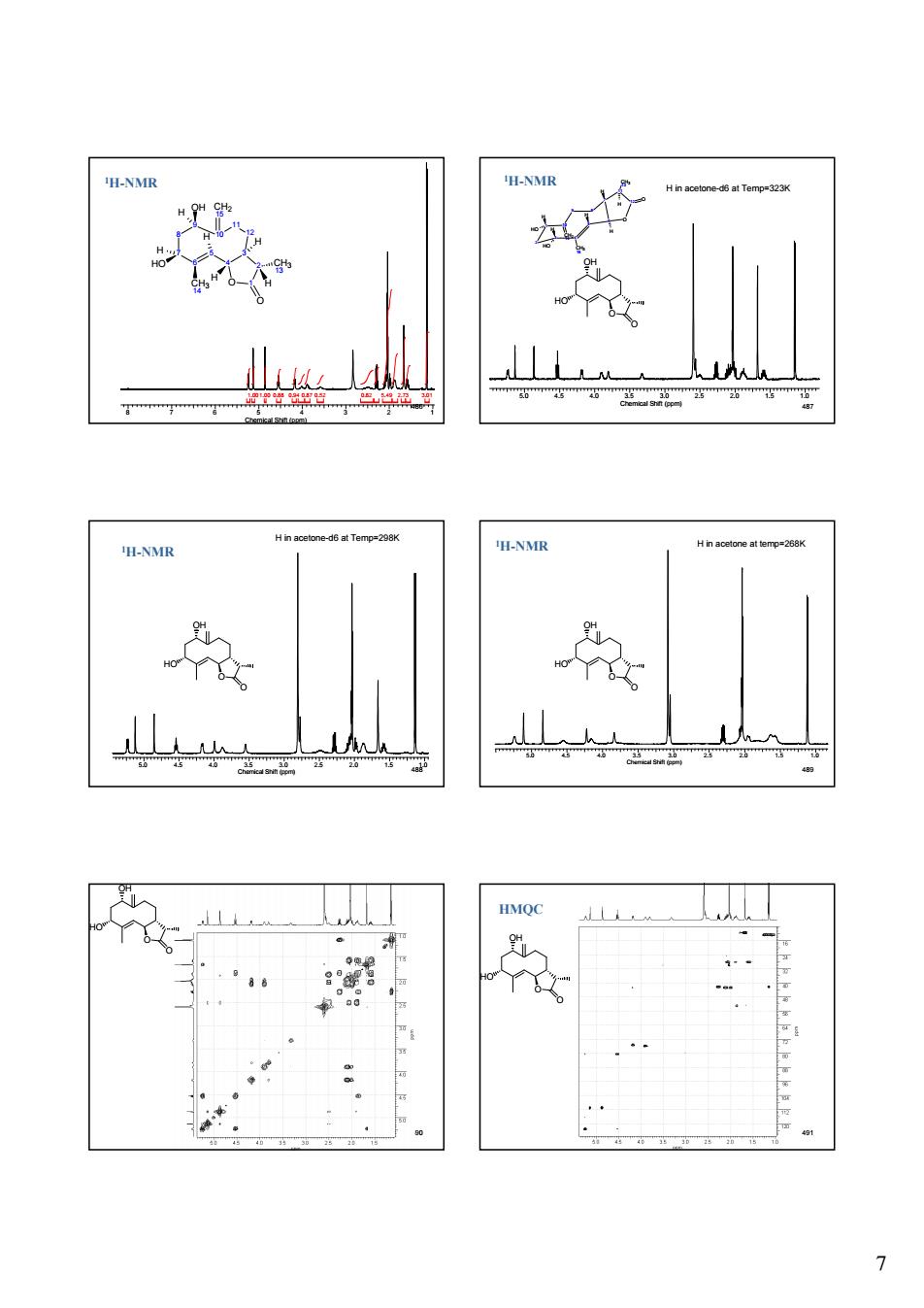
7 486 8 7 6 5 4 3 2 1 Chemical Shift (ppm) 1.00 1.00 0.88 0.94 0.87 0.52 0.82 5.49 2.73 3.01 10 5 9 6 8 7 12 3 4 2 O 1 O CH2 15 OH CH3 13 CH3 14 HO H H H H H H 11 1H-NMR 487 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) 2 1 3 10 4 5 CH3 15 H CH2 14 9 8 7 6 H HO H HO O 11 CH3 13 12 O H H H H in acetone-d6 at Temp=323K O OH HO O 1H-NMR 488 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) H in acetone-d6 at Temp=298K O OH HO O 1H-NMR 489 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) H in acetone at temp=268K O OH HO O 1H-NMR 490 O OH HO O 491 O OH HO O HMQC
HMBC NOESY
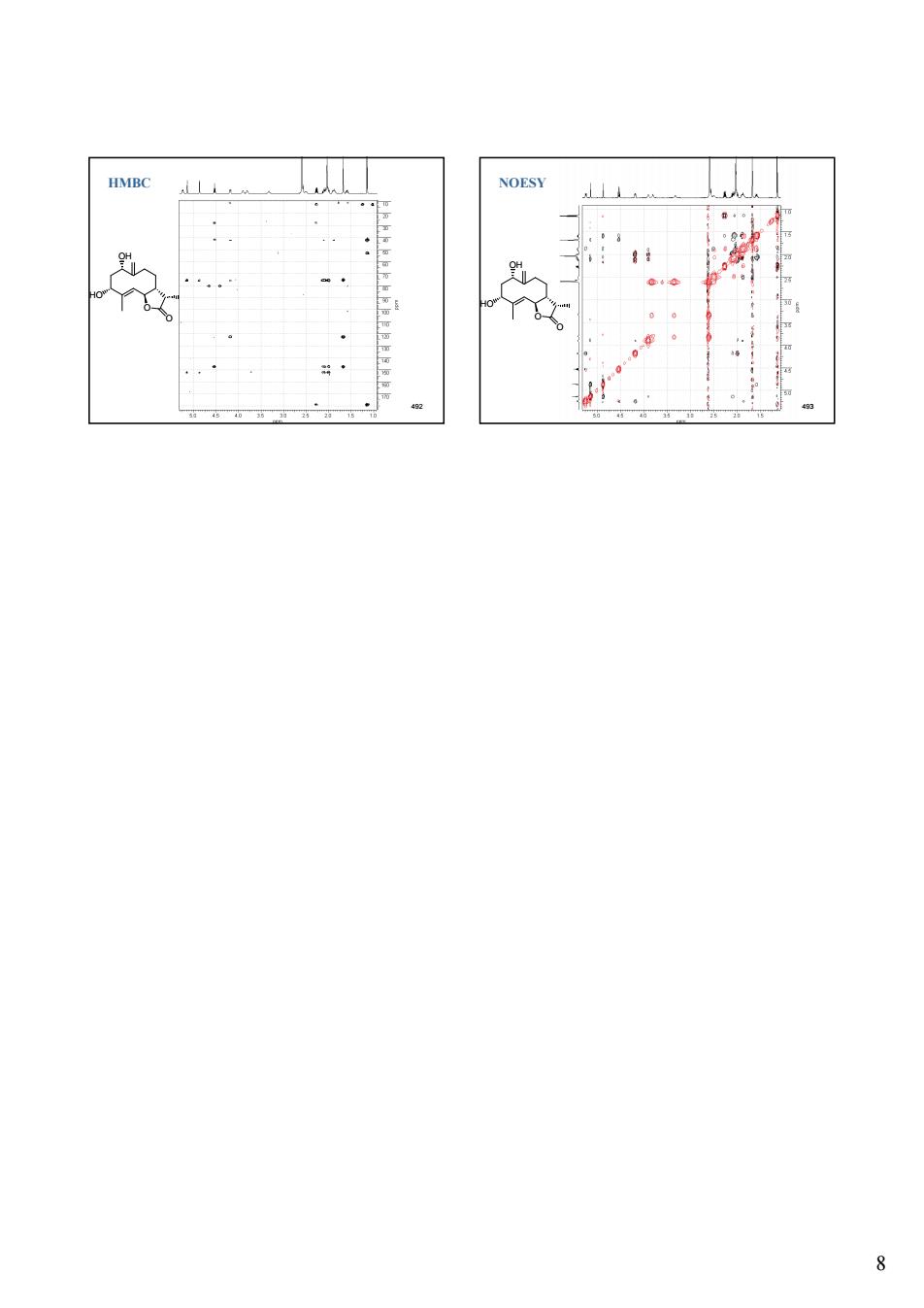
8 492 O OH HO O HMBC 493 O OH HO O NOESY