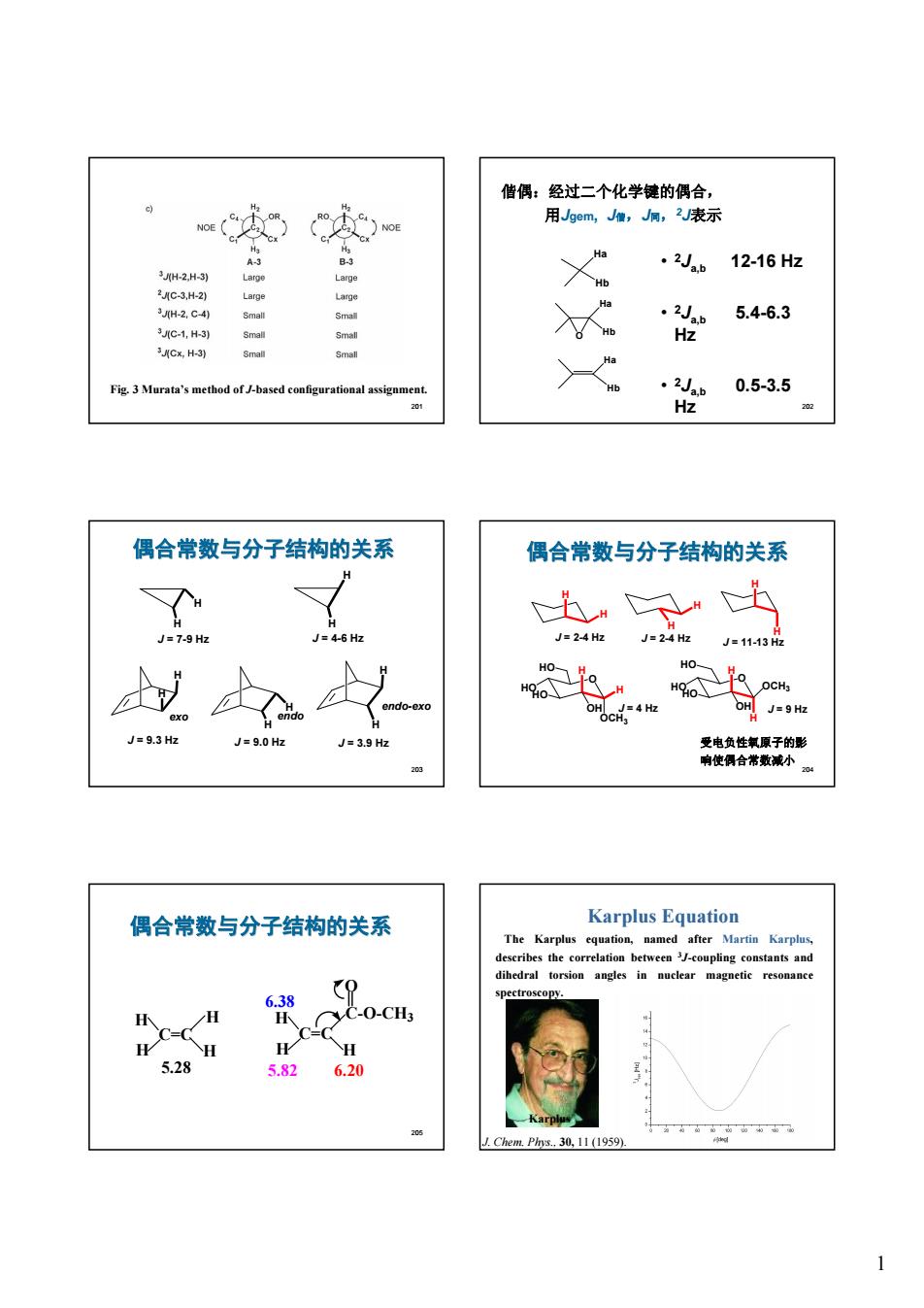
偕偶:经过二个化学健的偶合, 用gem,J,,2表示 12-16Hz 地 5.4-6.3 C-1,H-3) GH Fie.3 Marata's method of J-hased config 0.5-3.5 偶合常数与分子结构的关系 偶合常数与分子结构的关系 出 24J=24J=1143 半半 oc4 Je9.3Hz J=9.0 Hz J=39 偶合常数与分子结构的关系 Karplus Equation ihedral torsion angles in nuclear magnetic resonance c CO-CH3 H 5.28 CmPh,30,1山L959
1 201 Fig. 3 Murata’s method of J-based configurational assignment. 202 偕偶:经过二个化学键的偶合, 用Jgem, J偕,J同,2J表示 Ha Hb • 2Ja,b 12-16 Hz • 2Ja,b 5.4-6.3 Hz • 2Ja,b 0.5-3.5 Hz O Ha Hb Ha Hb 203 H H J = 7-9 Hz H H J = 4-6 Hz H H J = 9.3 Hz exo H H J = 9.0 Hz endo H H J = 3.9 Hz endo-exo 偶合常数与分子结构的关系 204 受电负性氧原子的影 响使偶合常数减小 偶合常数与分子结构的关系 J = 2-4 Hz H H J = 2-4 Hz H H J = 11-13 Hz H H O J = 4 Hz H OCH3 O J = 9 Hz H H OCH3 HO HO HO OH H OH HO HO HO 205 C=C H H H H C=C H H H O C-O-CH3 5.28 5.82 6.38 6.20 偶合常数与分子结构的关系 206 Karplus Equation The Karplus equation, named after Martin Karplus, describes the correlation between 3J-coupling constants and dihedral torsion angles in nuclear magnetic resonance spectroscopy. Karplus J. Chem. Phys., 30, 11 (1959)
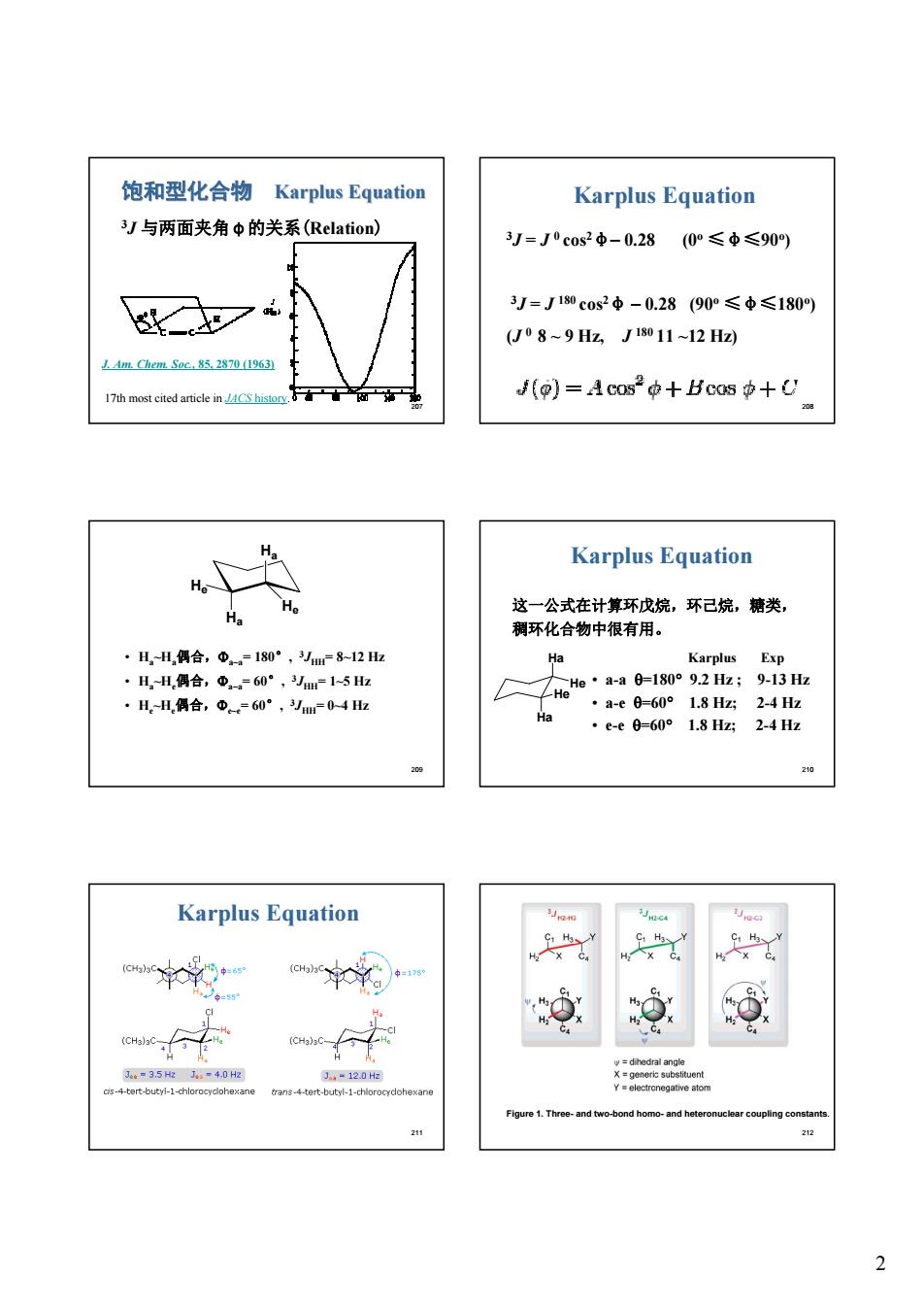
饱和型化合物Karplus Equation Karplus Equation 3J与两面夹角中的关系(Relation) J=J0c0s2中-0.28 (0°≤中≤90) 3j=J180c0s2中-0.28(90°≤中≤180) (U08~9Hz,J1011~12H2 J(p)=Acos°t+Bcos中+U Karplus Equation ·H,H偶合,Φ=180°,m812五 名 Karplus Exp ·H,-H,偶合,①-60°,-15z ·H-H偶合,中=60°,04 入e:aag=1s092H;,-g ·a-e0-60°1.8Hz2-4Hz ·e-e0-60°1.8Hz2-4Hz Karplus Equation nd home.and h 2 212 2
2 207 饱和型化合物 Karplus Karplus Equation 3J 与两面夹角φ的关系(Relation) J. Am. Chem. Soc., 85, 2870 (1963) 17th most cited article in JACS history. 208 Karplus Equation 3J = J 0 cos2φ- 0.28 (0o ≤φ≤90o) 3J = J 180 cos2φ - 0.28 (90o ≤φ≤180o) (J 0 8 ~ 9 Hz, J 180 11 ~12 Hz) 209 • Ha~Ha偶合,Fa~a= 180°, 3JHH= 8~12 Hz • Ha~He偶合,Fa~a= 60°, 3JHH= 1~5 Hz • He~He偶合,Fe~e= 60°, 3JHH= 0~4 Hz Ha Ha He He 210 这一公式在计算环戊烷,环己烷,糖类, 稠环化合物中很有用。 Karplus Exp • a-a q=180° 9.2 Hz ; 9-13 Hz • a-e q=60° 1.8 Hz; 2-4 Hz • e-e q=60° 1.8 Hz; 2-4 Hz Ha He He Ha Karplus Equation 211 Karplus Equation 212 Figure 1. Three- and two-bond homo- and heteronuclear coupling constants
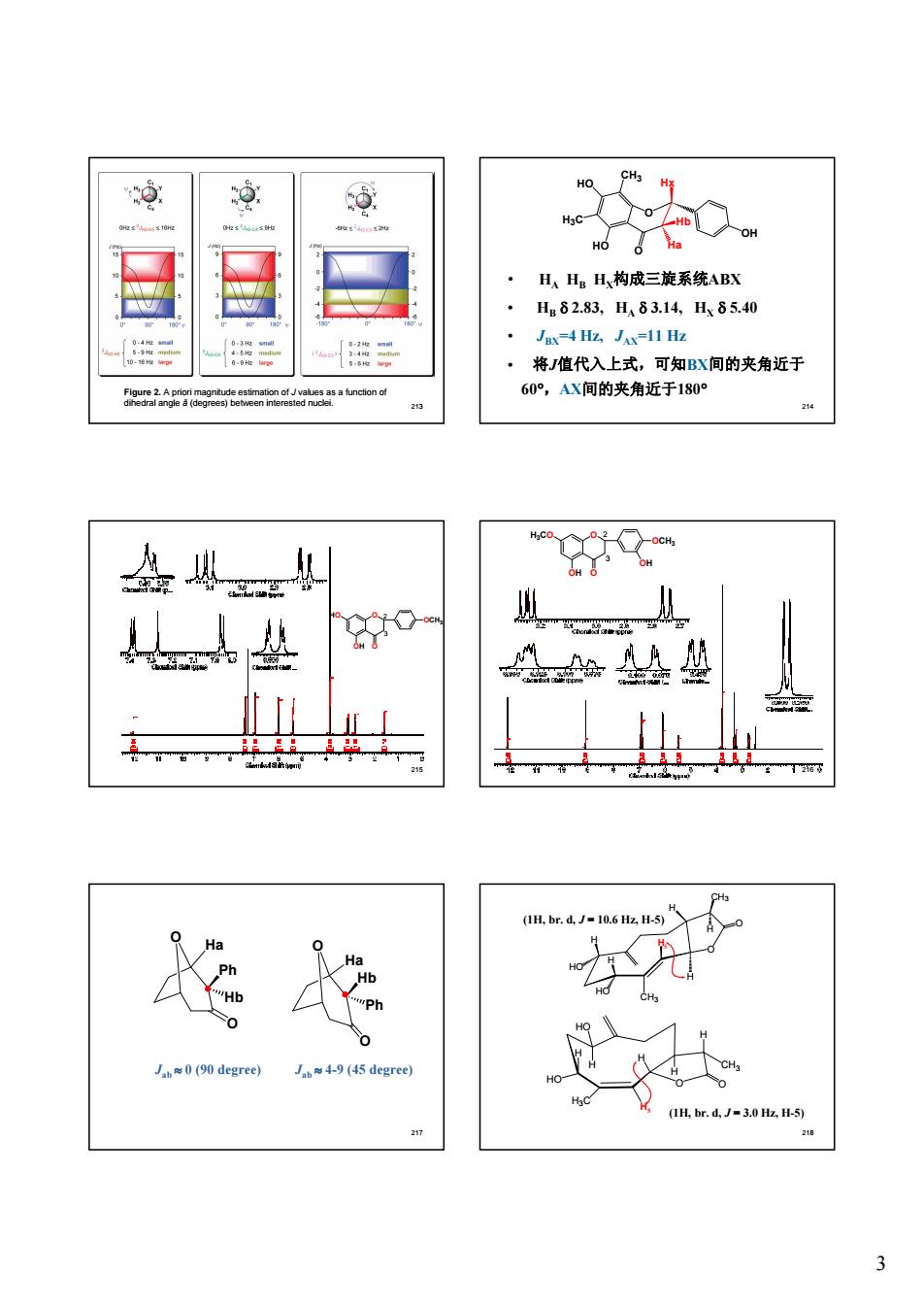
0 ⑤ 0 ·H、HgH构成三旋系统ABX H82.83,H83.14,H85.40 Jax=4 HZ JAx-11 Hz 将值代入上式,可知BX间的夹角近于 60°,AX间的夹角近于180° 盘从g 盐 护0 处给垫 的胶 9 degree) 49(45 degree (IH.br.d.J-3.0 Hz.H-5 27
3 213 Figure 2. A priori magnitude estimation of J values as a function of dihedral angle ã (degrees) between interested nuclei. 214 • HA HB HX构成三旋系统ABX • HB d 2.83, HA d 3.14, HX d 5.40 • JBX=4 Hz, JAX=11 Hz • 将J值代入上式,可知BX间的夹角近于 60°,AX间的夹角近于180° O O Ha Hx Hb CH3 H3C HO HO OH 215 O O OCH3 OH HO 2 3 216 O O OCH3 OH H3CO OH 3 2 217 O Ha Ph Hb O O Ha Hb Ph O Jab » 0 (90 degree) Jab » 4-9 (45 degree) 218 (1H, br. d, J = 10.6 Hz, H-5) (1H, br. d, J = 3.0 Hz, H-5) O CH3 O H H CH3 H H HO HO H H5 O CH3 O H H H3C H H HO HO H H5
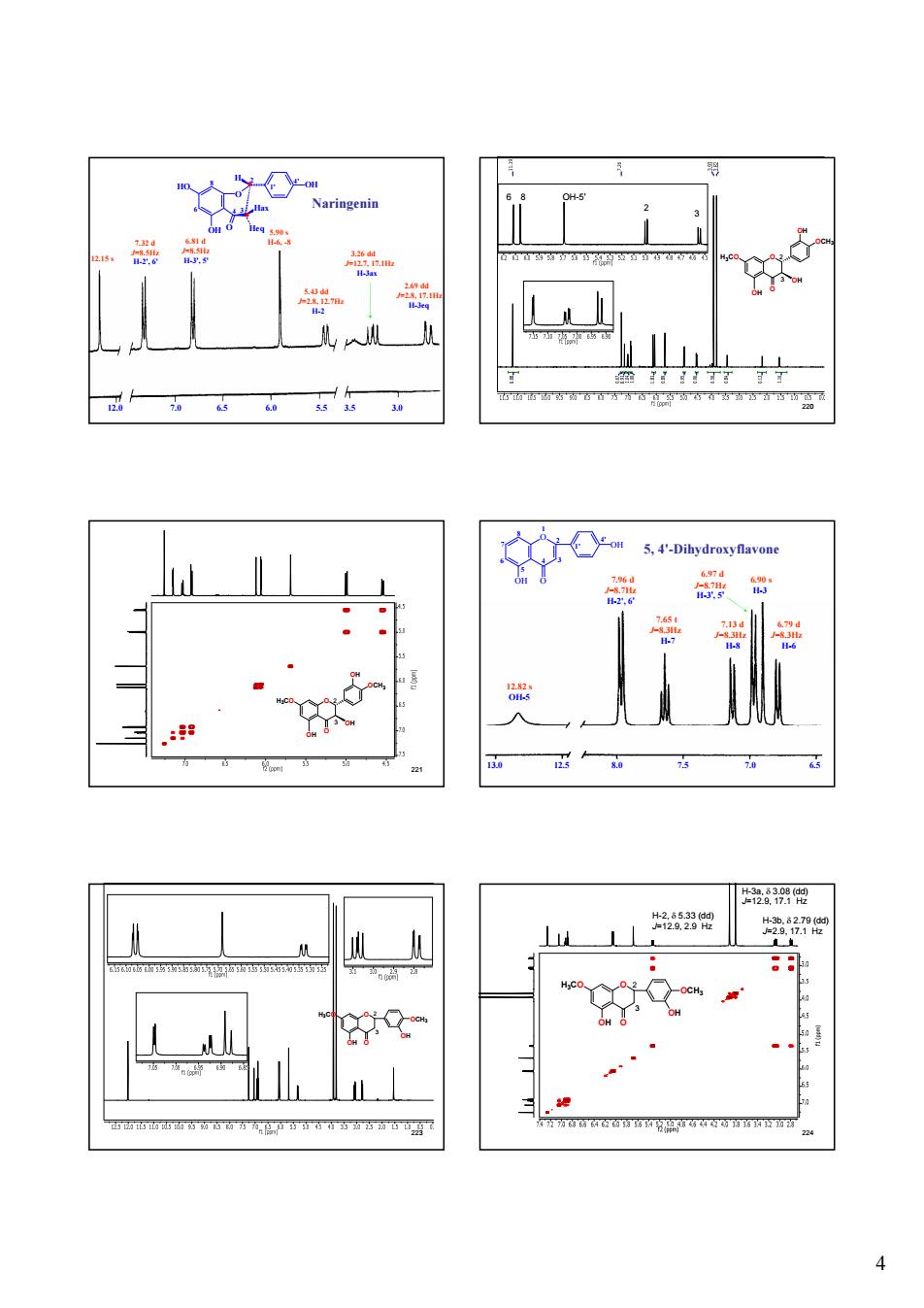
Naringenin 4
4 12.0 7.0 6.5 6.0 5.5 3.5 3.0 219 5.43 dd J=2.8, 12.7Hz H-2 3.26 dd J=12.7, 17.1Hz H-3ax 2.69 dd J=2.8, 17.1Hz H-3eq 12.15 s 5.90 s 7.32 d H-6, -8 J=8.5Hz H-2', 6' 6.81 d J=8.5Hz H-3', 5' Naringenin 2 6 4 3 8 4' 1' O OH O HO OH Heq Hax H 220 OH-5’ 2 3 6 8 O O OH OH OCH3 H3CO OH 2 3 221 O O OH OH OCH3 H3CO OH 2 3 222 3 7 5 4' 1' 8 6 4 2 1 O OH O OH 13.0 12.5 8.0 7.5 7.0 6.5 12.82 s OH-5 7.96 d J=8.7Hz H-2', 6' 7.65 t J=8.3Hz H-7 7.13 d J=8.3Hz H-8 6.97 d J=8.7Hz H-3', 5' 6.90 s H-3 6.79 d J=8.3Hz H-6 5, 4'-Dihydroxyflavone 223 O O OCH3 OH H3CO OH 3 2 224 H-2, d 5.33 (dd) J=12.9, 2.9 Hz H-3a, d 3.08 (dd) J=12.9, 17.1 Hz H-3b, d 2.79 (dd) J=2.9, 17.1 Hz O O OCH3 OH H3CO OH 3 2
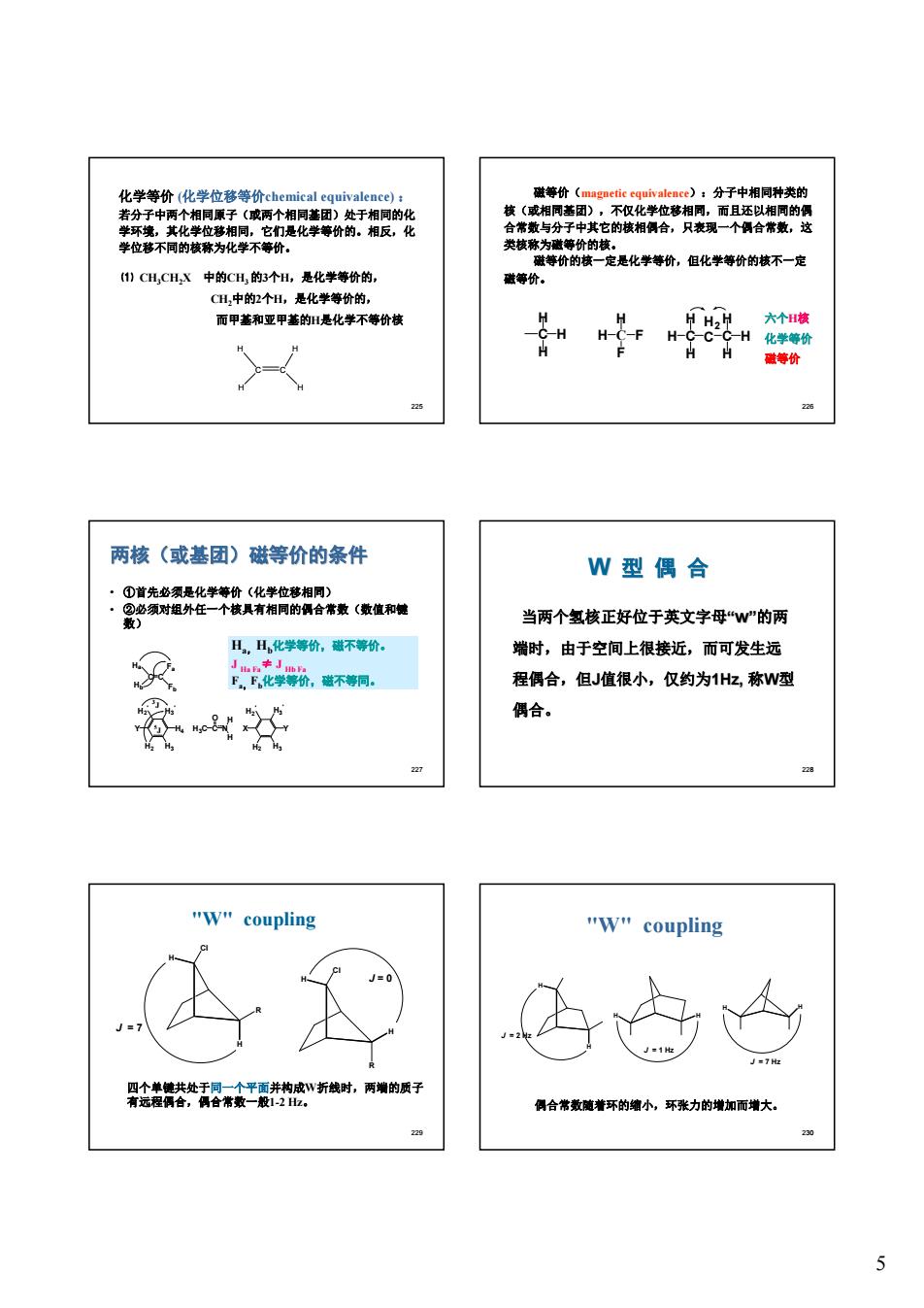
化学等价化学位价)的 分子中相同种类 就不- H,CHX中的C用的个,是化学等价的, 等价。 CH,中的2个H,是化学等价的, 而甲基和亚甲基的是化学不等价楼 两核(或基团)磁等价的条件 W型偶合 当两个氢核正好位于英文字母“W"的两 丑,H化学等价,蜚不等价。 端时,由于空间上很接近,而可发生远 ,营不铜 程偶合,但J值很小,仅约为1H化,称W型 偶合。 w"coupling "w"coupling 公 店 男验禁特活招老井的皮有时.青的质子 合常最葡着环的缩小,环张力的增加而增大 22m 5
5 225 化学等价 (化学位移等价chemical equivalence) : 若分子中两个相同原子(或两个相同基团)处于相同的化 学环境,其化学位移相同,它们是化学等价的。相反,化 学位移不同的核称为化学不等价。 C C H H H H ⑴ CH3CH2X 中的CH3 的3个H,是化学等价的, CH2中的2个H,是化学等价的, 而甲基和亚甲基的H是化学不等价核 226 磁等价(magnetic equivalence):分子中相同种类的 核(或相同基团),不仅化学位移相同,而且还以相同的偶 合常数与分子中其它的核相偶合,只表现一个偶合常数,这 类核称为磁等价的核。 磁等价的核一定是化学等价,但化学等价的核不一定 磁等价。 C H H H C H H F F C H2 C C H H H H H H 六个H核 化学等价 磁等价 227 两核(或基团)磁等价的条件 • ①首先必须是化学等价(化学位移相同) • ②必须对组外任一个核具有相同的偶合常数(数值和键 数) C C Ha Hb Fa Fb Y H2 ' H3 ' H4 H2 H3 3 J 5 J C O H3C N H H X Y H2 ' H3 ' H2 H3 Ha,Hb化学等价,磁不等价。 J Ha Fa≠ J Hb Fa Fa,Fb化学等价,磁不等同。 228 W 型 偶 合 当两个氢核正好位于英文字母“w”的两 端时,由于空间上很接近,而可发生远 程偶合,但J值很小,仅约为1Hz, 称W型 偶合。 229 "W" coupling H R Cl H H H Cl R J = 0 J = 7 四个单键共处于同一个平面并构成W折线时,两端的质子 有远程偶合,偶合常数一般1-2 Hz。 230 "W" coupling H H J = 2 Hz H J = 1 Hz H H H J = 7 Hz 偶合常数随着环的缩小,环张力的增加而增大

跨四个单键的折线型体系(W型) "w"coupling 水及为 跨五个单键的折线型体系 Ja,b-0 Hz ab=7 其它体系的远程偶合 Delayed COSY 之中产 gof signals which are very JBCA,J-based Configuration Analysis 固相核磁共振新技术 ,固相核藏共振技术可以实现对固体天然产物样品 的直按测定,但是所需样品量要大,所以在天然产 he ster 物分析中较少使用。 26 6
6 231 跨四个单键的折线型体系(W型) Hb Ha Hb Ha Hb Ha O H X O H 跨五个单键的折线型体系 N H O2N Ha Hc O Hb H H O 232 Ha Cl Hb Ha Cl N Hb O O N Ha Cl Hb O N Ha Cl Hb N O 4 Ja,b = 0 Hz 4 Ja,b = 7 Hz "W" coupling 233 其它体系的远程偶合 X Ha X Ha H Hb O b X Ha O Hb X Hb O Ha 234 Delayed COSY Delayed COSY allows linking of signals which are very weakly coupled (down to 0.1 Hz) Journal of Ethnopharm. 1991, 32, 103-110. 235 JBCA, J-based Configuration Analysis • NMR is the most useful method to elucidate the structures of natural products with a molecualar weight over 1000 since these compounds can hardly be crytalized. Murata had developed the method called "JBCA, J-based Configuration Analysis" which can be used to determine the stereochemistry of asymmetric carbon atoms residing in acyclic structures of natural products. • Murata,M., Matsuoka, S., Matsumori, N., Paul, G. K. and Tachibana, K. J. Am. Chem. Soc.. 121, 870-871 (1999). • Matsumori, N., Kaneno, D., Murata, M., Nakamura, H. and Tachibana, K. J. Org. Chem. 64, 866-876 (1999). 236 固相核磁共振新技术 • 固相核磁共振技术可以实现对固体天然产物样品 的直接测定,但是所需样品量要大,所以在天然产 物分析中较少使用

2DH-H相关谱H-HC0SY) 2DH-H相关谱(H-HC0SY) C0SY谱本身为正方形,当F1和F2谱宽 它的解方法是:取任一文作为出发点,通过它作垂 鼻是舟交变勤十韩,再计该之又作水 (diagonal peak)·对角线外的峰称为交叉峰 (cross peaks)或相关峰(correlated Paks)·每个相关峰或交叉峰反映两个峰组 要注意的是C0SY一般反映的潮合关系,但有时也会 间的糯合关系。COSY主要反映精合类系。 2 H-'H COSY H-H COSY H-H COSY 主要解决的问题 建立结构中存在 含无的与 的联系 H:C
7 237 2 D 1H-1H相关谱 (1H-1H COSY) • COSY 谱本身为正方形,当 F1和 F2谱宽 不等时则为矩形。正方形中有一条对角线(一 般为左下---右上)。对角线上的峰称为对角峰 (diagonal peak)。对角线外的峰称为交叉峰 ( cross peaks ) 或 相 关 峰 ( correlated peaks)。每个相关峰或交叉峰反映两个峰组 间的耦合关系。COSY 主要反映3J耦合关系。 238 2 D 1H-1H相关谱 (1H-1H COSY) • 它的解谱方法是:取任一交叉峰作为出发点,通过它作垂 线,会与某对角线峰及上方的氢谱中的某峰组相交,它们 即是构成此交叉峰的一个峰组。再通过该交叉峰作水平 线,与另一对角线峰相交,再通过该对角线峰作垂线,又 会与氢谱中的另一峰组相交,此即构成该交叉峰的另一峰 组。 • 要注意的是 COSY 一般反映的是3J 耦合关系,但有时也会 出现少数反映长程耦合的相关峰,另一方面,当3J 小时(如 两面角接近 90, 使3J很小),也可能没有相应的交叉峰。 239 1H-1H COSY 240 1H-1H COSY 2 3 4 1 5 241 主要解决的问题: 建立结构中存在偶 合关系的1H与1H 的联系 H O O CH3 H3C H 1 3 5 6 2 4 1H-1H COSY 3 2 4 5 6 242 H-14b H-14a H-3' H-2' H-10 H-9 H-20a H-20b H-5 H-13 H-3 H-1 H-2 H-6b H-6b H-6b H-6a H-6a Me-18 Ph-H H-20b H-3 Me-17 H-13 H-14a H-14b Me-18 H-10 O AcO H H OAc HO O 1 3 5 7 9 11 13 15 17 18 19 20 1' 2' 3' 10 Me-16 H-10 H-9 H-2' H-3
1H-NMR 端 Facts about chemical shifts and coupling constants 。 Preparation of NMR Samples NMR is of analysis is shorter instruments are always ready fo NMR instruments are quite versatile:with measurement,without any preceding the proper design,they can accommodate conditioning or calibration procedure solid,liquid or gas phase samples;even living being necessary. organisms can be measured.You will be making liquid samples
8 243 244 245 2 O 3 1 4 5 8 O 7 6 1' 6' 2' 5' 3' 4' O O 7' O O H H 246 Facts about chemical shifts and coupling constants: Chemical shifts in d ppm are independent of the strength of the applied field Bo and correspondingly, the instrument operating frequency. Chemical shifts in Hz are field and frequency dependent. Coupling constants J in Hz are field and frequency independent. Coupling constants J in d ppm are field and frequency dependent. 247 NMR is more robust and the time of analysis is shorter because the NMR instruments are always ready for measurement, without any preceding conditioning or calibration procedure being necessary. 248 Preparation of NMR Samples NMR instruments are quite versatile; with the proper design, they can accommodate solid, liquid or gas phase samples; even living organisms can be measured. You will be making liquid samples

Firstly we want to use a Deuterated solvent --It is important that the tho ugh deuterium is an NMR-active nucleu olution be free of p te,dust,libers,ef erent signal mnn0 Pack ap into a Pasteur pipette and wash with a small with deuterium.Normally,about99%of theH unt of solvent.Filter the solution into the atoms are replaced,leaving a residual proton NMR tube using a teat if necessary to force the a尚i,a sample through. 0.4-0.5 mL in a 5 mm NMR tube
9 249 . Firstly we want to use a Deuterated solvent. Although deuterium is an NMR-active nucleus, its signal comes at a very different signal than do the 1H nuclei you want to observe. We can prevent the solvent from "swamping" the sample signal by using solvents that have had the protons replaced with deuterium. Normally, about 98-99% of the H atoms are replaced, leaving a residual proton signal. This is actually quite useful as a chemical shift reference. 250 Sample filtration----It is important that the solution be free of precipitate, dust, fibers, etc. This can be achieved by filtering the sample. Make up slightly more sample than is required. Pack a small piece of cotton or glass wool tightly into a Pasteur pipette and wash with a small amount of solvent. Filter the solution into the NMR tube using a teat if necessary to force the sample through. 0.4-0.5 mL in a 5 mm NMR tube