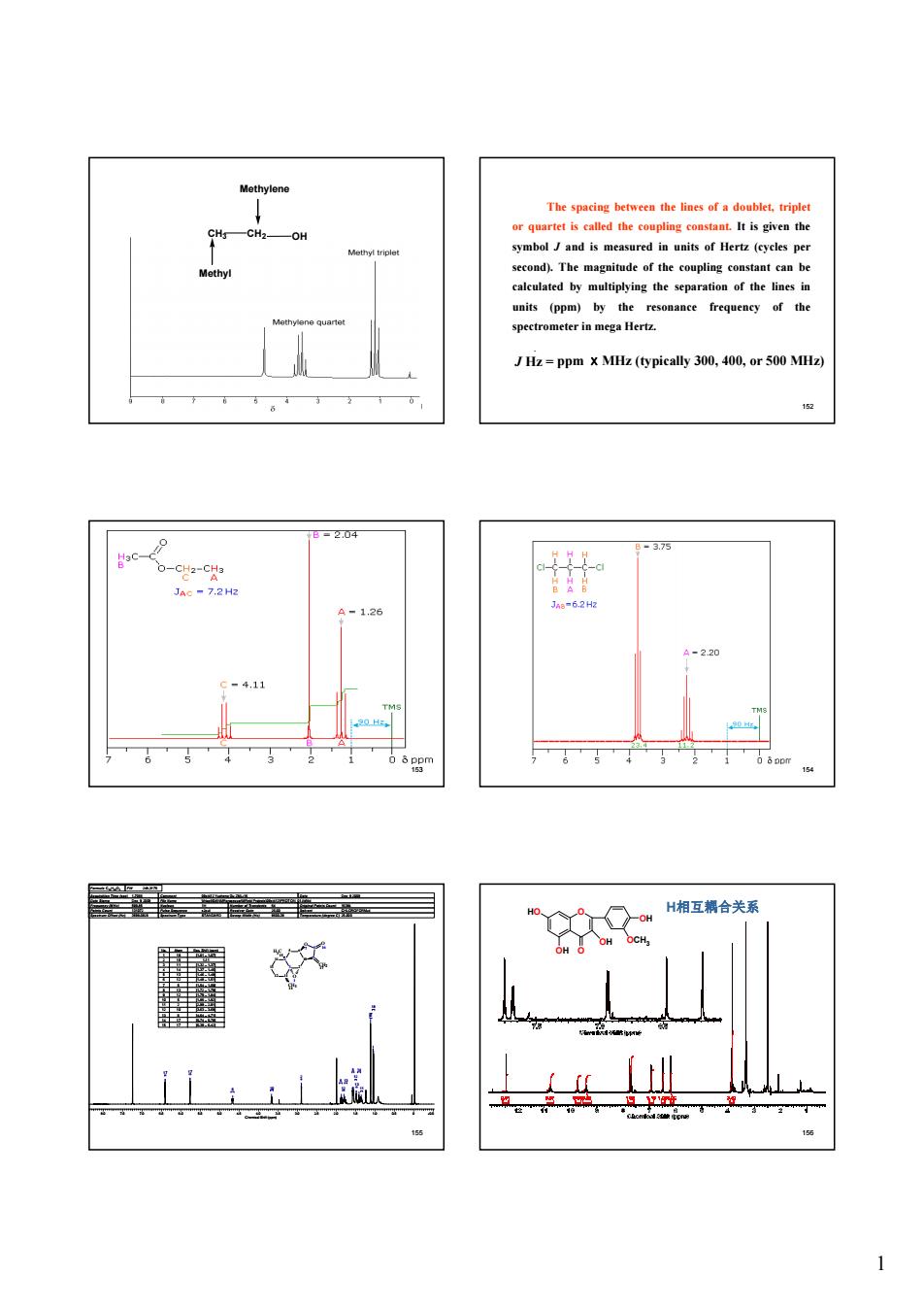
symbol Jand is measured in units of Hertz (cycles per second)The magnitude of the coupling costant can be Hz-ppmx MHz (typically 300,00, 20 A-126 -22 9-41 。 H相互合关 *
1 151 CH3 CH2 OH Methyl Methylene 152 The spacing between the lines of a doublet, triplet or quartet is called the coupling constant. It is given the symbol J and is measured in units of Hertz (cycles per second). The magnitude of the coupling constant can be calculated by multiplying the separation of the lines in units (ppm) by the resonance frequency of the spectrometer in mega Hertz. . J Hz = ppm ⅹMHz (typically 300, 400, or 500 MHz) 153 154 155 8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0 -0.5 Chemical Shift (ppm) 2 13 17 12 17 12 11 5 18 10 5 13 6 14 (15) Formula C15H20O3 FW 248.3175 Acquisition Time (sec) 1.7066 Comment 09st412 Yucheng Gu ZML-16 Date Dec 9 2009 Date Stamp Dec 9 2009 File Name \\friapfil04\NMRprocessor\MReid Projects\09st412\PROTON_01.fid\fid Frequency (MHz) 599.85 Nucleus 1H Number of Transients 64 Original Points Count 16384 Points Count 131072 Pulse Sequence s2pul Receiver Gain 26.00 Solvent CHLOROFORM-d Spectrum Offset (Hz) 3599.0928 Spectrum Type STANDARD Sweep Width (Hz) 9600.38 Temperature (degree C) 25.000 6 O 7 10 8 9 5 4 3 2 14 13 12 11 O 16 CH2 17 O 1 CH3 18 H3C 15 No. Atom Exp. Shift (ppm) 1 18 [1.01 .. 1.07] 2 15 1.11 3 11 [1.32 .. 1.37] 4 14 [1.37 .. 1.45] 5 13 [1.46 .. 1.49] 6 12 [1.48 .. 1.51] 7 5 [1.54 .. 1.59] 8 13 [1.72 .. 1.79] 9 12 [1.79 .. 1.84] 10 5 [1.85 .. 1.92] 11 2 [2.89 .. 2.91] 12 10 [3.63 .. 3.69] 13 6 [4.64 .. 4.71] 14 17 [5.74 .. 5.78] 15 17 [6.39 .. 6.42] 156 O O OH OH HO OH OCH3 H相互耦合关系
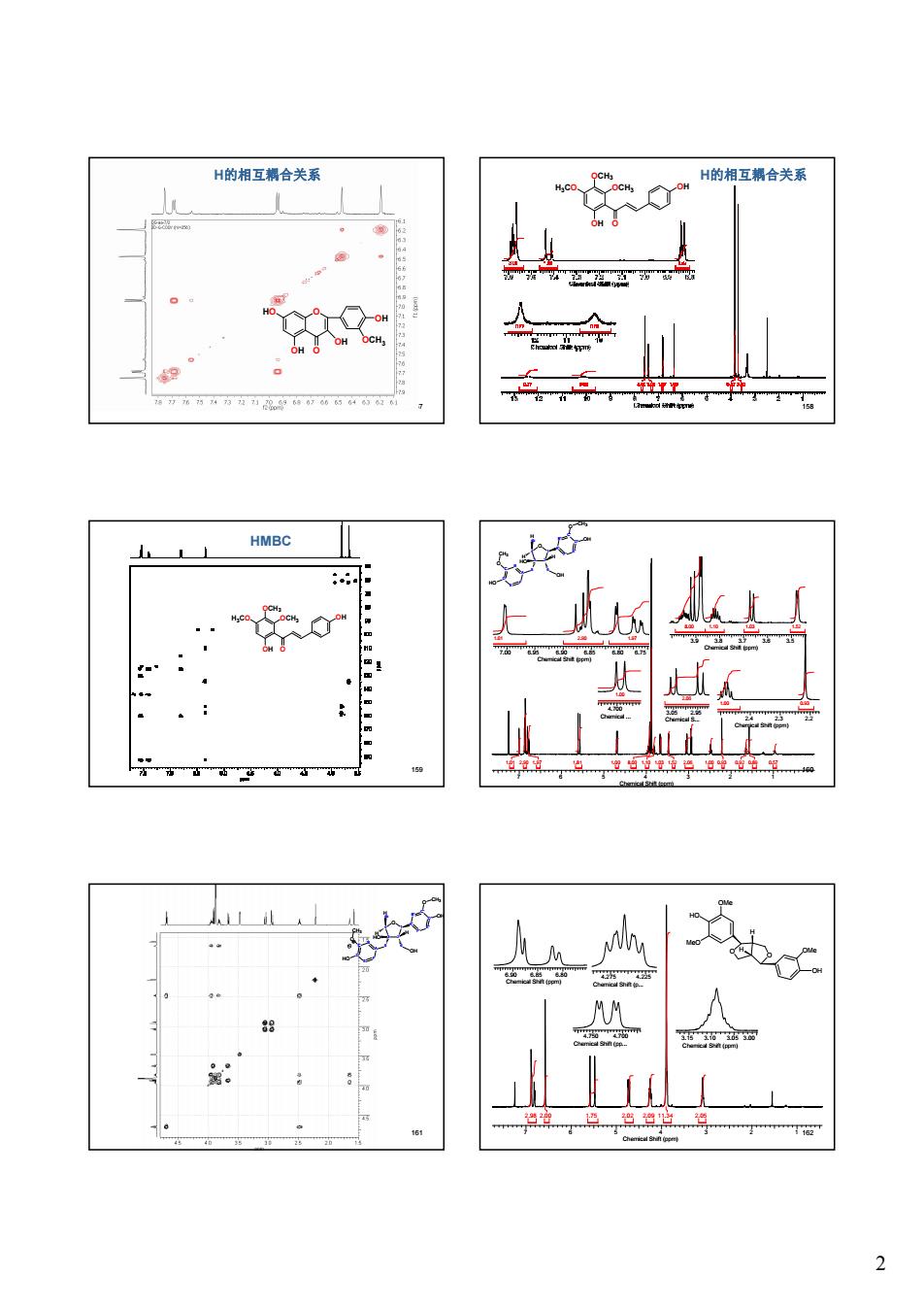
H的相互合关系 ,H的相互辆合关系 HMBC Ta
2 157 O O OH OH HO OH OCH3 H的相互耦合关系 158 OCH3 OH O H3CO OCH3 OH H的相互耦合关系 159 HMBC OCH3 OH O H3CO OCH3 OH 160 A F B E C D O CH3 5 3 4 2 6 1 O OH HO A' B' F' C' E' D' O OH CH3 HO H H 4b H 7 6 5 4 3 2 1 Chemical Shift (ppm) 1.01 2.90 1.97 1.81 1.00 8.00 1.10 1.03 1.52 2.06 1.00 0.93 0.92 0.89 0.57 7.00 6.95 6.90 6.85 6.80 6.75 Chemical Shift (ppm) 1.01 2.90 1.97 4.700 Chemical ... 1.00 3.9 3.8 3.7 3.6 3.5 Chemical Shift (ppm) 8.00 1.10 1.03 1.52 3.05 2.95 Chemical S... 2.06 2.4 2.3 2.2 Chemical Shift (ppm) 1.00 0.93 161 A F B E C D O CH3 5 3 4 2 6 1 O OH HO A' B' F' C' E' D' O OH CH3 HO H H 4b H 7 6 5 4 3 2 1 162 Chemical Shift (ppm) 2.98 2.00 1.75 2.02 2.09 11.34 2.05 6.90 6.85 6.80 Chemical Shift (ppm) 4.750 4.700 Chemical Shift (pp... 4.275 4.225 Chemical Shift (p... 3.15 3.10 3.05 3.00 Chemical Shift (ppm) O O H H MeO OH OMe HO OMe
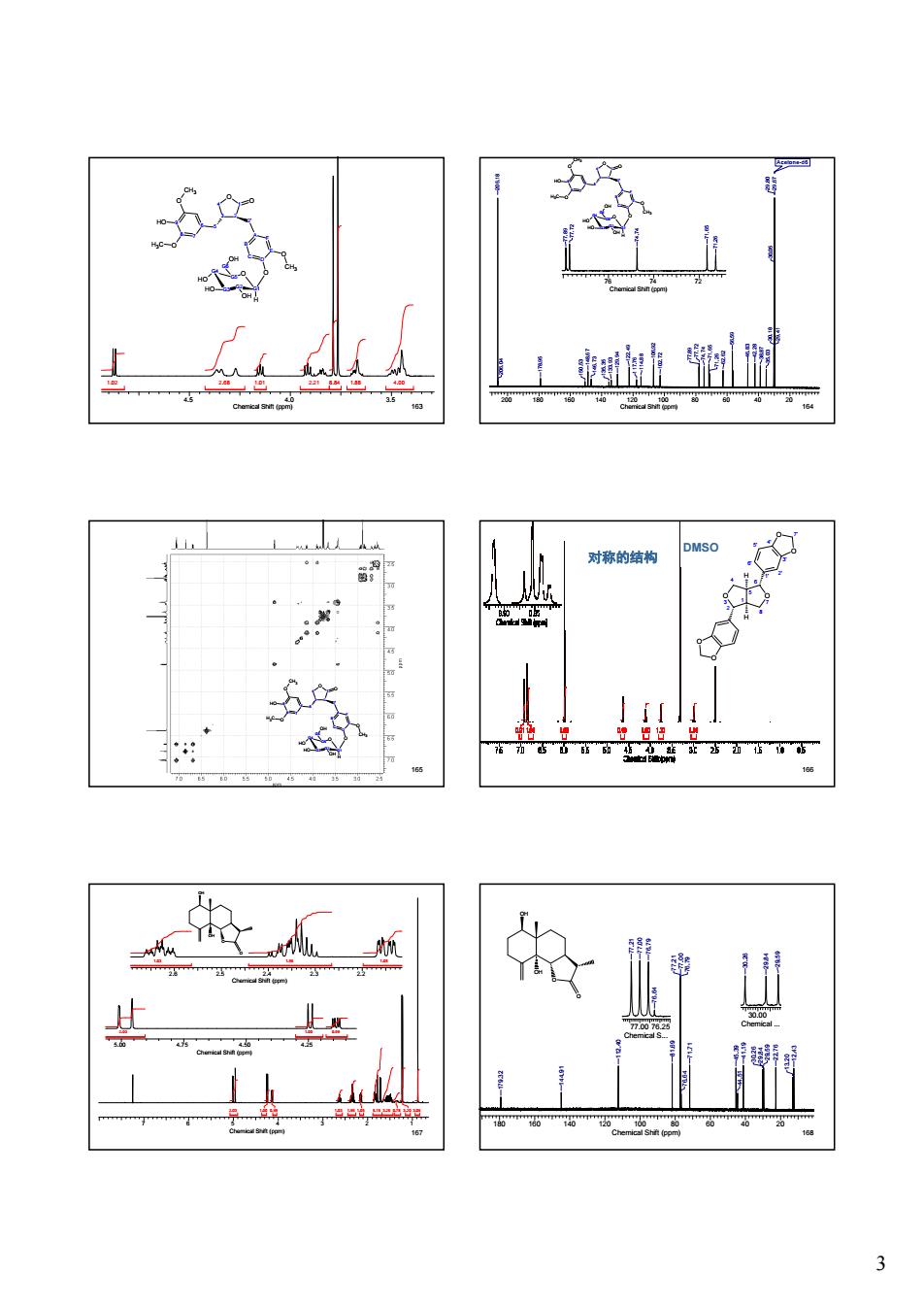
对称的结构 如 ☆ 上 边
3 163 4.5 4.0 3.5 Chemical Shift (ppm) 1.02 2.68 1.01 2.21 8.84 1.88 4.00 4 O 3 1 2 O 6 5 7 8 9 O HO O CH3 H3C 2' A F B E C D O O CH3 G4 G5 O G1 G2 G3 H OH HO HO G6 OH 164 200 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) Acetone-d6 29.41 29.80 29.67 30.05 30.18 35.03 38.87 46.83 42.28 56.59 62.62 71.26 71.65 74.74 77.72 77.89 102.72 106.92 114.88 117.76 122.49 129.94 133.93 135.35 146.73 148.67 150.53 178.95 206.04 206.18 76 74 72 Chemical Shift (ppm) 71.26 71.65 74.74 77.72 77.89 4 O 3 1 2 O 6 5 8 7 9 O HO O CH3 H3C 2' A F B E C D O O CH3 G4 G5 O G1 G2 G3 H OH HO HO G6 OH 165 4 O 3 1 2 O 6 5 8 7 9 O HO O CH3 H3C 2' A F B E C D O O CH3 G4 G5 O G1 G2 G3 H OH HO HO G6 OH 166 2 O 3 1 4 5 8 O 7 6 1' 6' 2' 5' 3' 4' O O 7' O O H H DMSO 对称的结构 167 7 6 5 4 3 2 1 Chemical Shift (ppm) 2.03 1.00 0.99 1.03 1.96 1.05 5.15 3.26 0.78 3.20 3.05 5.00 4.75 4.50 4.25 Chemical Shift (ppm) 2.03 1.00 0.99 2.6 2.5 2.4 2.3 2.2 Chemical Shift (ppm) 1.03 1.96 1.05 O OH OH O 168 O OH OH O 180 160 140 120 100 80 60 40 20 Chemical Shift (ppm) 12.43 13.20 29.59 22.76 30.26 29.84 41.19 44.51 45.39 71.71 76.64 76.79 77.21 77.00 81.69 112.40 144.91 179.32 77.00 76.25 Chemical S... 76.64 77.21 77.00 76.79 30.00 Chemical ...29.59 30.26 29.84
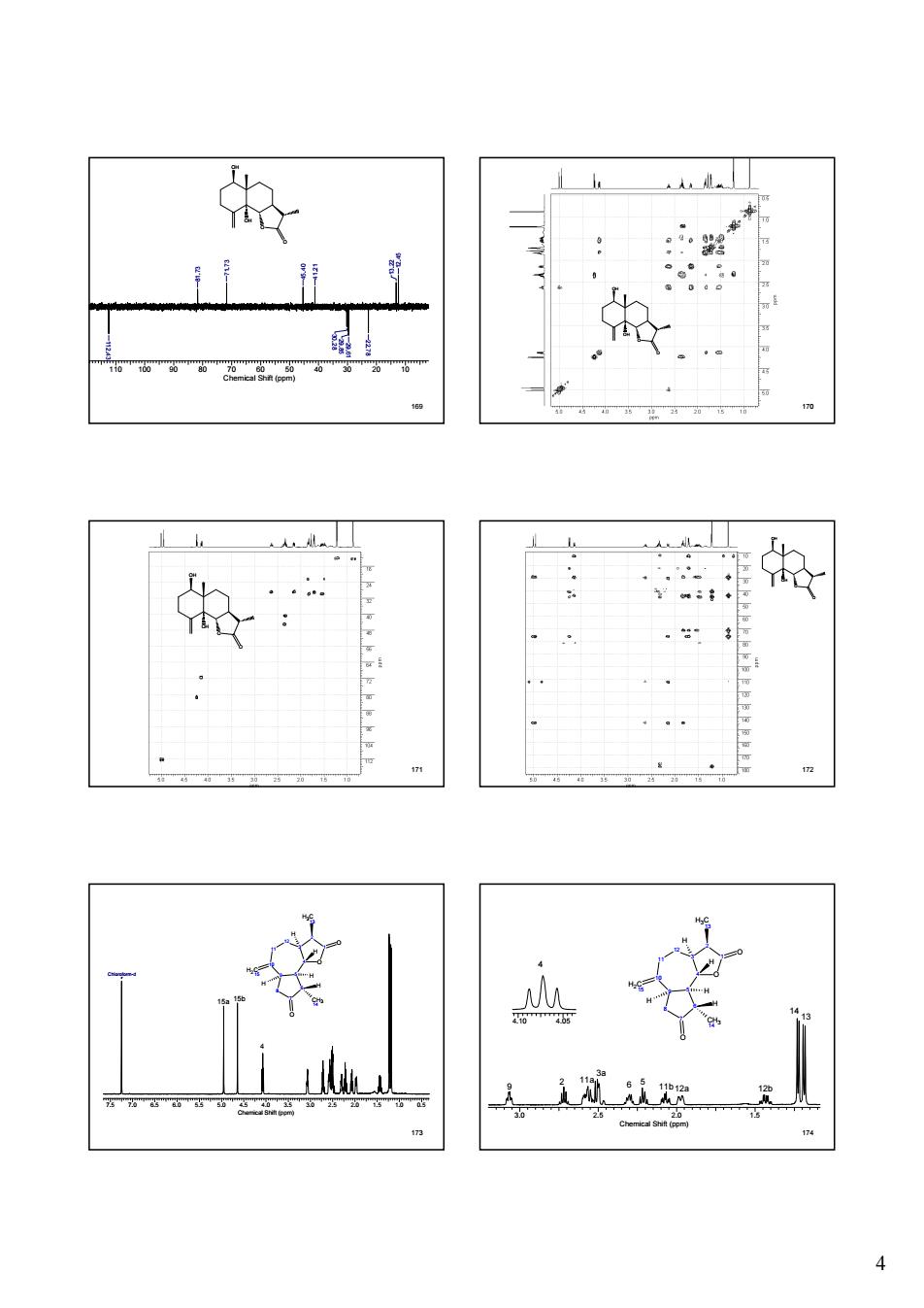
☆ 人从
4 169 O OH OH O 110 100 90 80 70 60 50 40 30 20 10 Chemical Shift (ppm) 12.45 13.22 22.78 29.61 29.85 30.28 45.40 41.21 71.73 81.73 112.43 170 O OH OH O 171 O OH OH O 172 O OH OH O 173 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 Chemical Shift (ppm) Chloroform-d 3 2 4 1 O 12 11 5 9 10 6 7 8 H3C 13 O H2C 15 CH3 14 H H H H H O 15a 15b 4 174 4.10 4.05 3 2 4 1 O 12 11 5 9 10 6 7 8 H3C 13 O H2C 15 CH3 14 H H H H H O 3.0 2.5 2.0 1.5 Chemical Shift (ppm) 4 9 2 11a 3a 6 5 11b12a 12b 14 13
C门门门0M L
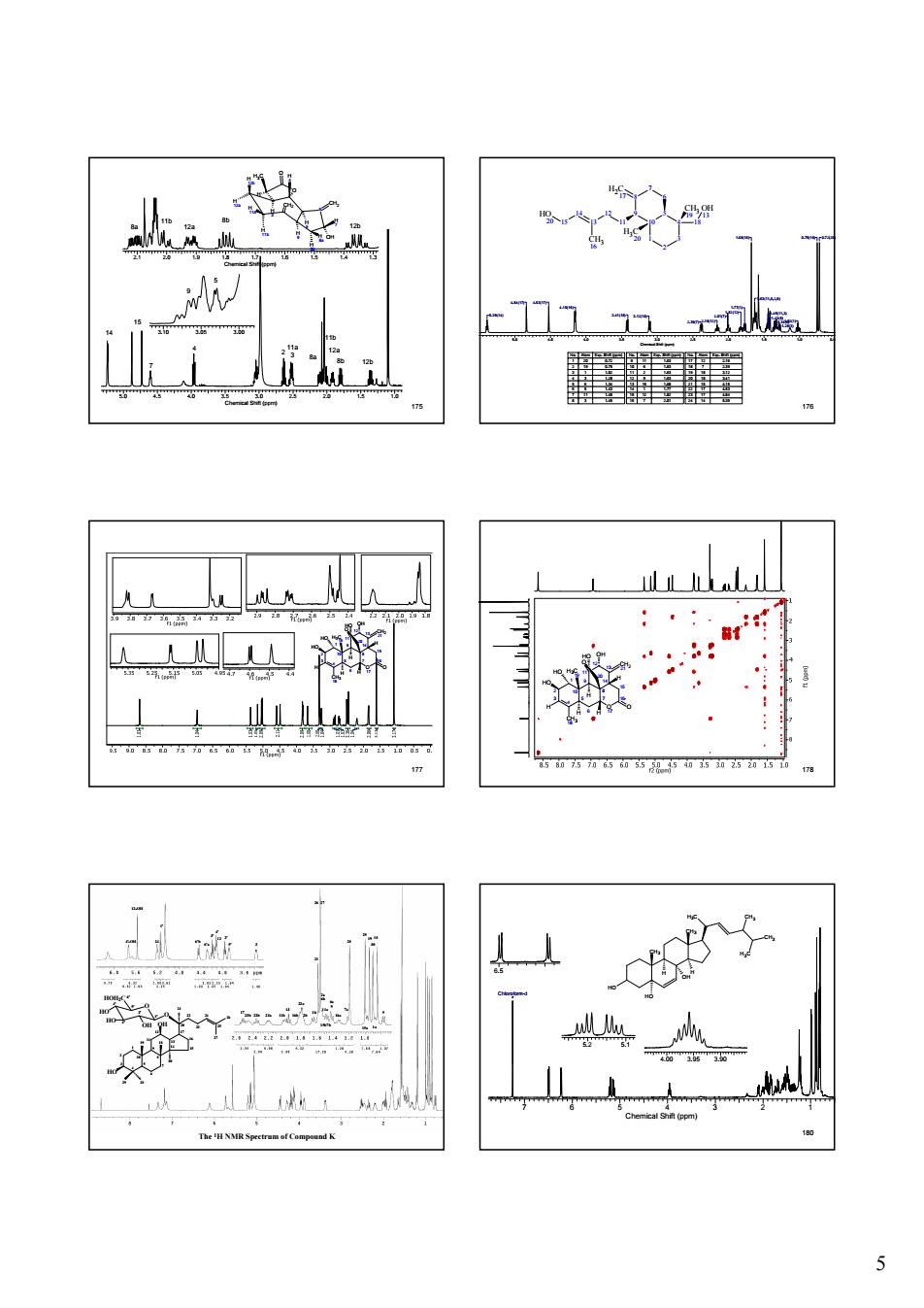
5 175 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) 14 3.10 3.05 3.00 15 7 4 2 11a 3 9 5 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 Chemical Shift (ppm) 8a 11b 12a 12b 8b 8a 11b 8b 12a 12b H 4 O O H 12b H 12a H 3 H 5 H 9 H CH2 11a H 11b 6 CH2 OH H 7 H 8a H 8b H3C H 176 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 Chemical Shift (ppm) 0.75(19) 0.72(20) 1.02(1) 1.28(3) 1.34(6) 1.43(5) 1.45(11,3) 1.63(11,6,2,9) 1.68(16) 1.77(1) 1.82(12) 2.01(7) 2.16(12) 2.39(7) 3.12(18) 3.41(18) 4.15(15) 4.84(17) 4.53(17) 5.39(14) 6 5 7 10 8 9 4 3 2 1 H3C 20 CH3 19 18 OH 13 H2C 17 11 12 13 14 CH3 16 15 HO 20 No. Atom Exp. Shift (ppm) 1 20 0.72 2 19 0.75 3 1 1.02 4 3 1.28 5 6 1.34 6 5 1.43 7 11 1.45 8 3 1.45 No. Atom Exp. Shift (ppm) 9 11 1.63 10 6 1.63 11 2 1.63 12 9 1.63 13 16 1.68 14 1 1.77 15 12 1.82 16 7 2.01 No. Atom Exp. Shift (ppm) 17 12 2.16 18 7 2.39 19 18 3.12 20 18 3.41 21 15 4.15 22 17 4.53 23 17 4.84 24 14 5.39 177 10 5 1 4 2 3 8 7 9 6 15 16 14 O 17 13 12 11 HO CH3 18 H3C 19 CH2 21 OH O O 20 H H H HO HO H H 178 10 5 1 4 2 3 8 7 9 6 15 16 14 O 17 13 12 11 HO CH3 18 H3C 19 CH2 21 OH O O 20 H H H HO HO H H 179 The 1H NMR Spectrum of Compound K 4'-OH 12-OH 6' 5' 4' 3' 2' 1' 29 28 19 18 30 21 27 26 25 24 23 22 20 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 O HOH2C OH HO OH O HO HO 24 1' 6'b 6'a 3' 4' 12 2' 5' 3 1723b 22b 23a 11b 13 16b 22a 2b 1b 21 26 27 2a 6b 11a 15b7b 6a 9 16a 7a 28 15a 1a 5 29 19 30 18 180 7 6 5 4 3 2 1 Chemical Shift (ppm) Chloroform-d 6.5 5.2 5.1 4.00 3.95 3.90 H3C HO CH3 CH3 OH H H CH3 CH3 H3C HO
小 COSY E H in acetone-d6 at Temp-3231 核磁共振氢谱中构象的变化 U 6
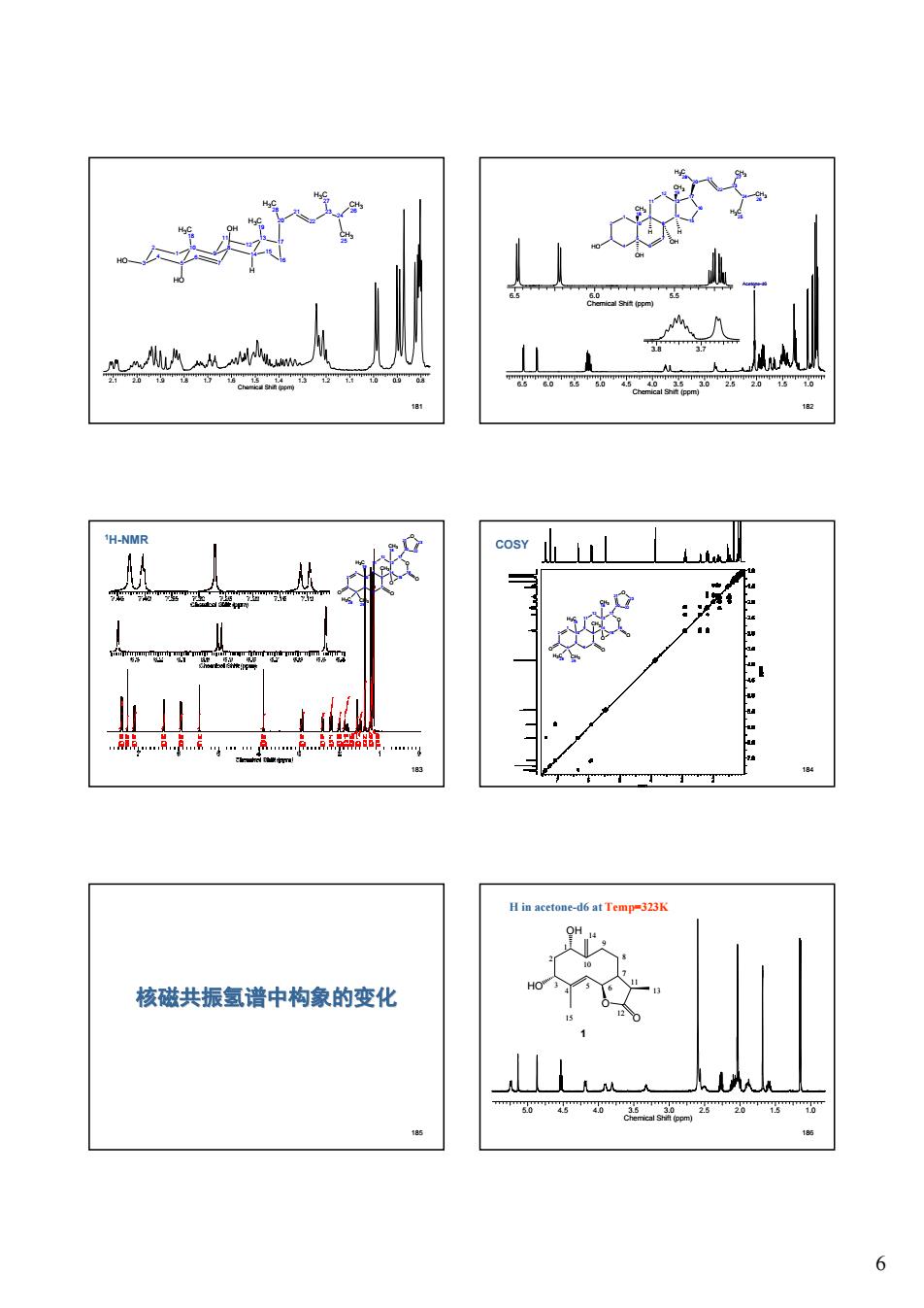
6 181 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 Chemical Shift (ppm) 3 2 1 10 5 4 9 8 7 6 11 12 13 14 17 16 15 H3C 18 H3C 19 HO HO OH 20 21 H3C 28 22 23 24 H3C 27 CH3 25 CH3 26 H 182 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) Acetone-d6 6.5 6.0 5.5 Chemical Shift (ppm) 3.8 3.7 13 14 17 10 16 8 9 5 12 15 11 1 4 20 7 2 3 6 H3C 28 HO CH3 19 CH3 18 21 22 OH H H 23 24 CH3 26 CH3 27 H3C 25 OH 183 1H-NMR 10 5 1 4 2 3 8 7 9 6 13 14 12 11 O 16 17 15 20 21 22 O 23 CH3 18 CH3 H3C 19 H3C 28 CH3 29 O O O O 184 COSY 10 5 1 4 2 3 8 7 9 6 13 14 12 11 O 16 17 15 20 21 22 O 23 CH3 18 CH3 H3C 19 H3C 28 CH3 29 O O O O 185 核磁共振氢谱中构象的变化 186 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) H in acetone-d6 at Temp=323K O OH HO O 1 4 7 10 13 15 2 3 5 6 8 9 11 12 14 1
H in acetone-d6 at Temp-263 "NN3MU,30MH 新水新此 人M N-NMR C 500 MH 透 山小 X东动 i 五i 410℃,500 >
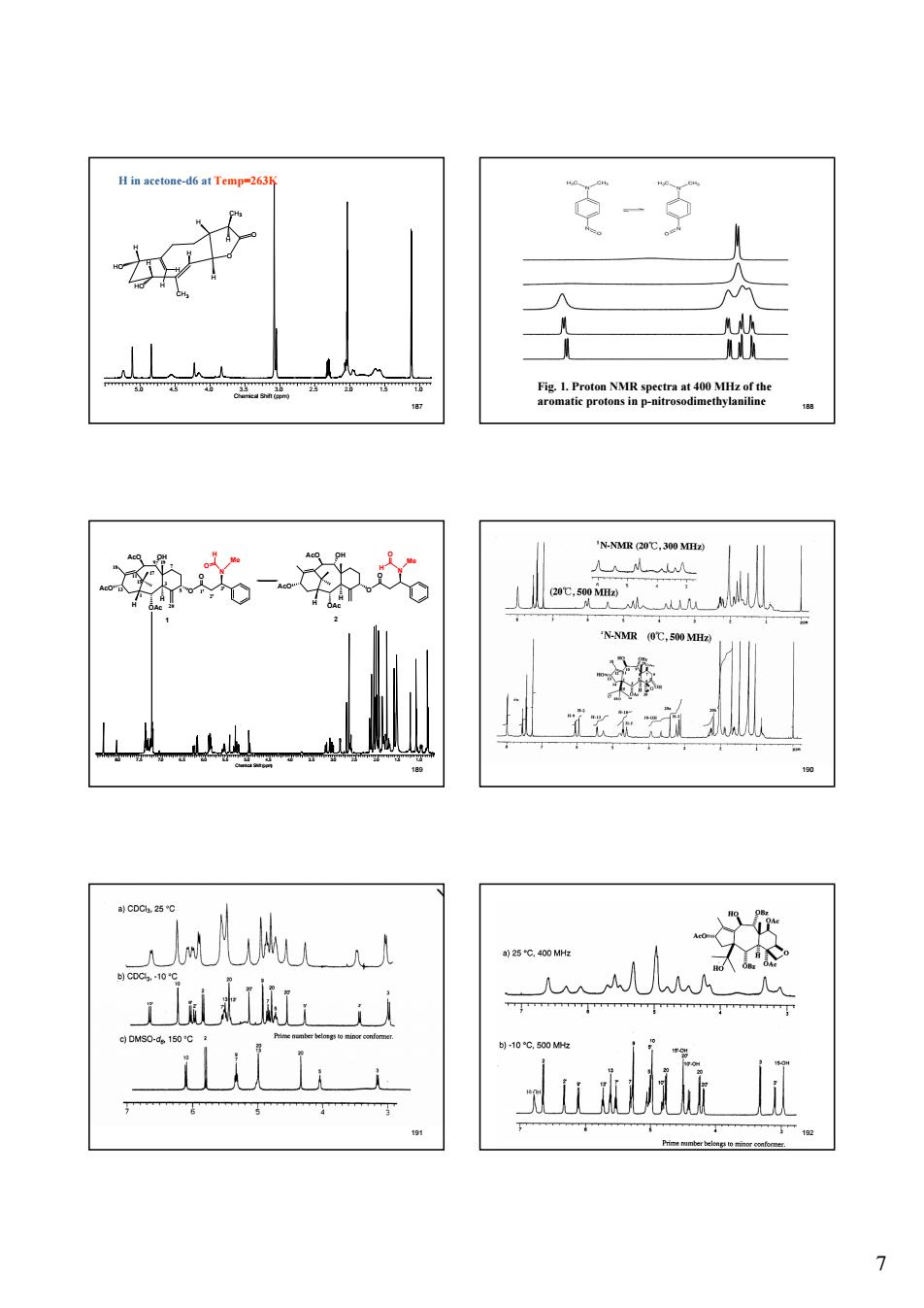
7 187 H in acetone-d6 at Temp=263K 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) O CH3 O H H CH3 H H HO HO H H H H 188 Fig. 1. Proton NMR spectra at 400 MHz of the aromatic protons in p-nitrosodimethylaniline 189 H OAc H O AcO 1 OH AcO O N Me H O H OAc H O AcO 2 OH AcO O N Me H O 1 3 5 7 9 11 13 15 17 1' 2' 3' 18 19 20 8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 Chemical Shift (ppm) 190 191 192

20℃ -40℃ 2R:0 60C 80C 20℃ 偶合常数J(Coupling Constants) 偶合常数J(Coupling Constants) ·自旋一自旋偶合产生峰的裂分,面每组峰中峰与 峰之间的距离叫阀合常数(Coupling constants), ,(3)饱和烃化合物,自旋偶合传递作用一般 用表示单位业 不超过3个化学健: ·特点: ,(4)有的相隔4个或5个键也可看到偶合裂 山不受外磁场影响,外磁场变化,值不变: ·(2)质子之间产生的偶合裂分,值<20H: 分,相隔4个键以上的偶合裂分称远程偶合 (Long-range coupling). the 偶合常数与分子结构的关系 红will be thes bether the spectrum is meas 根据相互干扰氢核之间相隔键数的多少, 将偶合作用分为: 偕偶(Geminal coupling)也称偕质子偶 合,同碳质子偶合。 ·邻偶(Vicinal coupling) sor geminal coupling ,远程偶合(Long-range coupling)
8 193 194 195 偶合常数 J (Coupling Constants) (Coupling Constants) • 自旋¾自旋偶合产生峰的裂分,而每组峰中峰与 峰之间的距离叫偶合常数 (Coupling constants), 用J表示单位Hz. • 特点: • (1) J不受外磁场影响,外磁场变化,J值不变; • (2) 质子之间产生的偶合裂分,J值< 20 Hz; 196 • (3) 饱和烃化合物,自旋偶合传递作用一般 不超过3个化学键; • (4) 有的相隔4个或5个键也可看到偶合裂 分,相隔4个键以上的偶合裂分称远程偶合 (Long-range coupling)。 偶合常数 J (Coupling Constants) (Coupling Constants) 197 H-C-H, two sigma bonds or geminal coupling H-C-C-H, three sigma bonds or vicinal coupling Because of the mechanism of J coupling, the magnitude is field independent: coupling constants in Hertz will be the same whether the spectrum is measured at 300 MHz or 500 MHz. Coupling constants range in magnitude from 0 to 20 Hz. Observable coupling will generally occur between hydrogen nuclei that are separated by no more than three sigma bonds. 198 偶合常数与分子结构的关系 • 根据相互干扰氢核之间相隔键数的多少, 将偶合作用分为: • 偕 偶 (Geminal coupling)也 称 偕 质 子 偶 合,同碳质子偶合。 • 邻偶 (Vicinal coupling) • 远程偶合 (Long-range coupling)

女太或安 器 22222 Fig.I Murata's method of J-based configurational assignmer FMurata'method of Jbased 9
9 199 Fig. 1 Murata’s method of J-based configurational assignment. N. Matsumori, D. Kaneno, M. Murata, H. Nakamura and K. Tachibana, J. Org. Chem., 1999, 64, 866-876. Fig. 2 Murata’s method of J-based configurational assignment. 200