
第11章天然药物的研究与开发 双语教学读物 Introduction to Natural Medicines R D What a Natural Product? Natural products are organic compounds produced by many microbes,plants and marine invertebrates.It is thought by some that toxic natural products are produced by the host as adaptations to deterpredators (chemical defense)or to compete for space in their native environments (alleopathy).Some natural products also exhibit biological activityrelevant to human physiology and disease states.Many terrestrial natural products have found use in medicine (gmorphine from the opium poppy,digitalis glycosides from foxglove)and agriculture (e.g.the potent insect anti-feedant azadarichtin from Neem treeoil).however exploration of marine natural products is relatively recent Isolation of pure natural products from various sources .Structure identification of natural products using a wide variety of NMR and MS techniques. Analysis by all types of chromatography eg.TLC.PC.HPLC.CCete. Chemistry of Pharmaceutical Natural Products Welcome to the course in natural product chemistry.The course aims at giving a general overview of the chemistry of natural products with the emphasis on secondary metabolites (primary metabolites are commonly regarded as the domain of the biochemists).With the biosynthetic pathways as a basis,knowledge of the major classes of natural products and their chemical and structural characteristics will be provided.The study of pharmaceutical natural
第 11 章 天然药物的研究与开发 双语教学读物 Introduction to Natural Medicines R & D What a Natural Product? Natural products are organic compounds produced by many microbes, plants and marine invertebrates. It is thought by some that toxic natural products are produced by the host as adaptations to deterpredators (chemical defense) or to compete for space in their native environments (alleopathy). Some natural products also exhibit biological activityrelevant to human physiology and disease states. Many terrestrial natural products have found use in medicine (e.g. morphine from the opium poppy, digitalis glycosides from foxglove) and agriculture (e.g. the potent insect anti-feedant azadarichtin from Neem treeoil), however exploration of marine natural products is relatively recent. · Isolation of pure natural products from various sources · Structure identification of natural products using a wide variety of NMR and MS techniques. · Analysis by all types of chromatography eg. TLC, PC, HPLC, CC etc. Chemistry of Pharmaceutical Natural Products Welcome to the course in natural product chemistry. The course aims at giving a general overview of the chemistry of natural products with the emphasis on secondary metabolites (primary metabolites are commonly regarded as the domain of the biochemists). With the biosynthetic pathways as a basis, knowledge of the major classes of natural products and their chemical and structural characteristics will be provided. The study of pharmaceutical natural
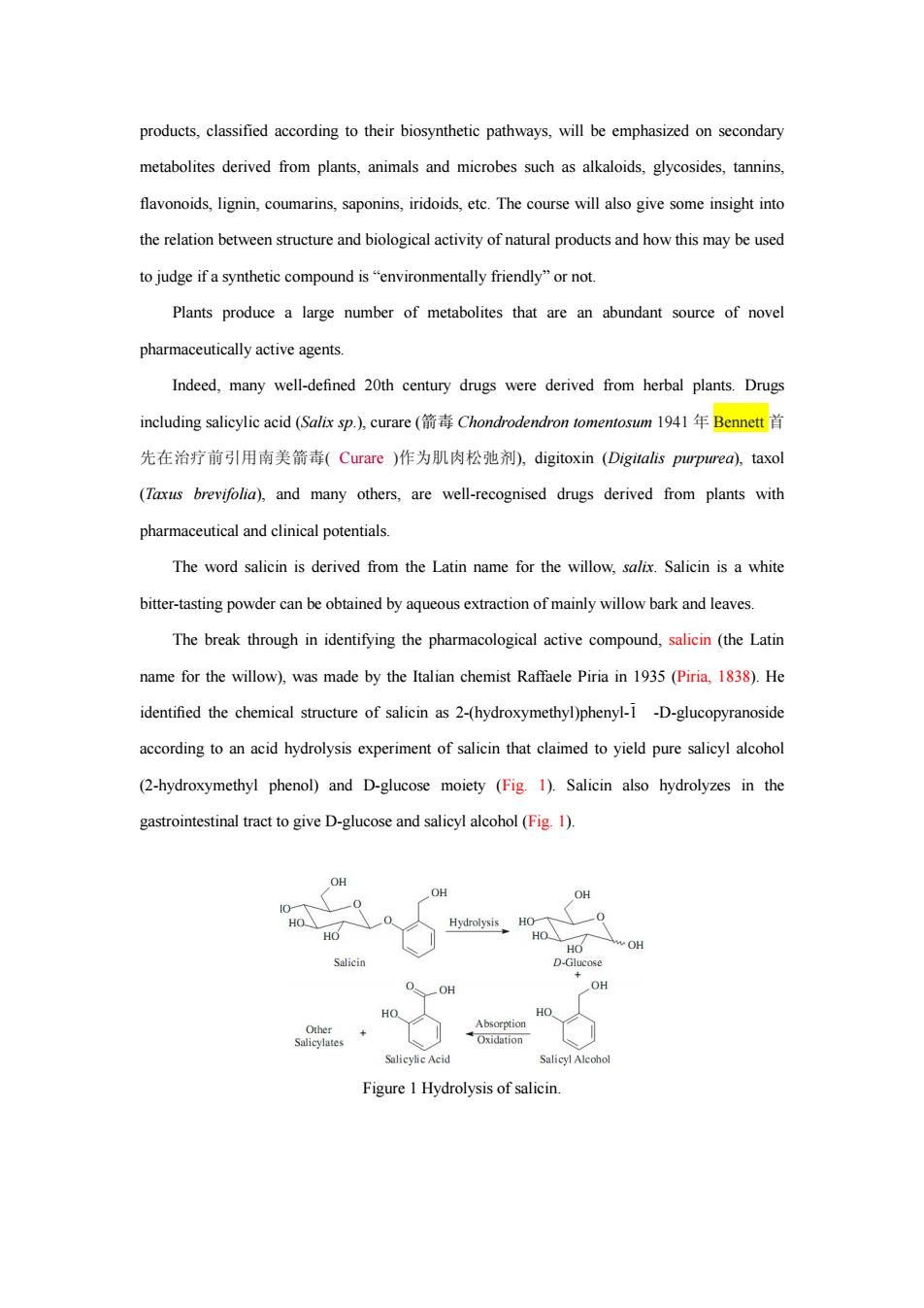
products,classified according to their biosynthetic pathways,will be emphasized on secondary metabolites derived from plants,animals and microbes such as alkaloids,glycosides,tannins, flavonoids,lignin,coumarins,saponins,iridoids,ete.The course will also give some insight into therelation and of natural productsand howthis maybeused to judge if a synthetic compound is"environmentally friendly"or not. Plants produce a large number of metabolites that are an abundant source of novel pharmaceutically active agents. Indeed,many well-defined 20th century drugs were derived from herbal plants.Drugs including salicylic acid(Salix sp.,curare(箭毒Chondrodendron tomentosum194l年Bennett首 先在治疗前引用南美箭毒(Curare)作为肌肉松弛剂,digitoxin(Digitalis purprea),taxol (Txs brevifolia)and many others.are well-recognised drugs derived from plants with pharmaceutical andotenia The word salicin is derived from the Latin name for the willow,salix.Salicin is a white bitter-tasting powder can be obtained by aqueous extraction of mainly willow bark and leaves. The break through in identifying the pharmacologica active(the Latin name for the willow),was made by the Italian chemist Raffaele Piria in 1935(Piria,1838).He identified the chemical structure of salicin as 2-(hydroxymethyl)phenyl-1-D-glucopyranoside according to an acid hydrolysis experiment of salicin that claimed to yield pure salicy alcohol (2-hydroxymethyl phenol)and D-glucose moiety (Fig.1).Salicin also hydrolyzes in the gastrointestinal tract to give D-glucose and salicyl alcohol (Fig 1). OH HO 0 HO Figure I Hydrolysis of salicin
products, classified according to their biosynthetic pathways, will be emphasized on secondary metabolites derived from plants, animals and microbes such as alkaloids, glycosides, tannins, flavonoids, lignin, coumarins, saponins, iridoids, etc. The course will also give some insight into the relation between structure and biological activity of natural products and how this may be used to judge if a synthetic compound is “environmentally friendly” or not. Plants produce a large number of metabolites that are an abundant source of novel pharmaceutically active agents. Indeed, many well-defined 20th century drugs were derived from herbal plants. Drugs including salicylic acid (Salix sp.), curare (箭毒 Chondrodendron tomentosum 1941 年 Bennett 首 先在治疗前引用南美箭毒( Curare )作为肌肉松弛剂), digitoxin (Digitalis purpurea), taxol (Taxus brevifolia), and many others, are well-recognised drugs derived from plants with pharmaceutical and clinical potentials. The word salicin is derived from the Latin name for the willow, salix. Salicin is a white bitter-tasting powder can be obtained by aqueous extraction of mainly willow bark and leaves. The break through in identifying the pharmacological active compound, salicin (the Latin name for the willow), was made by the Italian chemist Raffaele Piria in 1935 (Piria, 1838). He identified the chemical structure of salicin as 2-(hydroxymethyl)phenyl-1 -D-glucopyranoside according to an acid hydrolysis experiment of salicin that claimed to yield pure salicyl alcohol (2-hydroxymethyl phenol) and D-glucose moiety (Fig. 1). Salicin also hydrolyzes in the gastrointestinal tract to give D-glucose and salicyl alcohol (Fig. 1). Figure 1 Hydrolysis of salicin

- of salicyl aldehyde into their acid derivative Figure 3 Acetylation of salicylic acid. Piria,R.Sur des nouveaux produits extraits de la salicin.C.R.Acad.Sci.Paris 1838,6,620-624. Jassem G Mahdi.Medicinal potential of willow:A chemical perspective of aspirin discovery. Journal of Saudi Chemical Sociery1.14.317-322. Literature projects 1.Taxol and its Derivatives,a Plant-Derived Anticancer Agent 2.Natural Products in Different Seasons. 3.Ginseng Is the Advertisment Empty Words or has it Some Substance? 4.Insects as a Source for Raw Materials. 5.Etoposide(VP-26),a Plant-Derived Anticancer Agent 6.Artemisinin,an Antimalarial Natural Product. 7.The stories of Aspirin,Morphine,Quinine,Penicillin
Figure 2 Oxidation of salicyl aldehyde into their acid derivative. Figure 3 Acetylation of salicylic acid. Piria, R. Sur des nouveaux produits extraits de la salicin. C.R. Acad. Sci. Paris 1838, 6, 620–624. Jassem G. Mahdi. Medicinal potential of willow: A chemical perspective of aspirin discovery. Journal of Saudi Chemical Society 2010, 14, 317–322. Literature projects 1. Taxol and its Derivatives, a Plant-Derived Anticancer Agent 2. Natural Products in Different Seasons. 3. Ginseng. Is the Advertisment Empty Words or has it Some Substance? 4. Insects as a Source for Raw Materials. 5. Etoposide (VP-26), a Plant-Derived Anticancer Agent 6. Artemisinin, an Antimalarial Natural Product. 7. The stories of Aspirin, Morphine, Quinine, Penicillin

Success in Drug Research An compound with an 10 Chemical Space of Organic Molecules 00 A hit is no a lead ead is no Acandidate not a drug A drug is no success Asuccessful ua is luck PartI The Use of Natural Products as Drugs in History Natural products appear to have been used to cure illnesses almost since the beginning of time.Possibly the oldest known recipes for such cures are found on a set of 660 clay tablets from the Mesopotamian civilization dating to the third millennium B.C.E.These tablets contain a list of more than a thousand plants used for medicinal purposes. Natural medicines were being used in China at about the same time.A famous text dating to about 1000 B.C.E.,the Huang Ti Nei Ching Su Wen (Yellow Emperor's canon of internal medicine),is regarded as the oldest record of traditional Chinese medical techniques and describes treatments that were used as far back as about 2500 B.C.E.The oldest Chinese book containing recipes for herbal treatments is Shen Nung Pen Ts'ao Ching(Shen Nung's catalog of herbs),dating to about 1000 B.C.E. Relatively little progress was made in the West in the treatment of disease from the rise of Christianity to the end of the Middle Ages,to some extent because illness
Part I The Use of Natural Products as Drugs in History Natural products appear to have been used to cure illnesses almost since the beginning of time. Possibly the oldest known recipes for such cures are found on a set of 660 clay tablets from the Mesopotamian civilization dating to the third millennium B.C.E. These tablets contain a list of more than a thousand plants used for medicinal purposes. Natural medicines were being used in China at about the same time. A famous text dating to about 1000 B.C.E., the Huang Ti Nei Ching Su Wen (Yellow Emperor’s canon of internal medicine), is regarded as the oldest record of traditional Chinese medical techniques and describes treatments that were used as far back as about 2500 B.C.E. The oldest Chinese book containing recipes for herbal treatments is Shen Nung Pen Ts’ao Ching (Shen Nung’s catalog of herbs), dating to about 1000 B.C.E. Relatively little progress was made in the West in the treatment of disease from the rise of Christianity to the end of the Middle Ages, to some extent because illness

was regarded as punishment for one's sins.As a result,prayer and the hope for miracles were frequently the only methods available for the cure of disease.During the Renaissance,however,a renewed interest in the use of plant materials(usually herbs)sprang up in Europe.The first pharmacopoeia in the modern era,written by the German botanist Valerius Cordus (February 18.1515-September 25.1544),was published posthumously in 1546.(A pharmacopoeia is a list of drugs and medicines. with a description of the ilnesses for which they are useful and instructions for their preparation.) Valerius Cordus (Valeris Cors (Febuary 11515-Seplember 25,154)Gem erhals in history.He is also widely credited with developing a method for synthesizing ether(which h called by the poetic Latin name oleton dlci rimriofi,or "sweet oil of vitriol"). Cordus wrote prolifically,and identified and described several new plant species and vanetes he plant senus Cordia(紫草科破布木属)is named for him. Cordus's Dispensatorium was soon followed by other pharmacopoeia in other parts of Germany and other countries of Europe.The first such book in the United States,the Litit Pharmacopoeia,was published by Dr. William Brown in 1778 for use in military hospitals during the American Revolution. As in the modern world,plant materials were used not only to treat disease but also for other purposes,the most important of which was to produce hallucinogenic, psychedelic,or other"out-of-body"experiences.Many cultures throughout history have made the use of such materials an integral part of their religious ceremonies.For One of the most widely used of all illegal drugs is marijuana,which comes from the camnabis sativa plant.(Ted Kinsman/Photo Researchers,Inc.)example,ancient Hindu documents assert that the hallucinogenic effects of marijuana were fi rst discovered by the god Shiva,who ate leaves of the plant and found them very refreshing.Thereafter. the plant was routinely used in many Hindu ceremonies,usually in a form known as bhang.In the New World,the peyote cactus has been used in religious ceremonies for at least 10,000 years
was regarded as punishment for one’s sins. As a result, prayer and the hope for miracles were frequently the only methods available for the cure of disease. During the Renaissance, however, a renewed interest in the use of plant materials (usually herbs) sprang up in Europe. The first pharmacopoeia in the modern era, written by the German botanist Valerius Cordus (February 18, 1515 – September 25, 1544), was published posthumously in 1546. (A pharmacopoeia is a list of drugs and medicines, with a description of the illnesses for which they are useful and instructions for their preparation.) Valerius Cordus (Valerius Cordus (February 18, 1515 – September 25, 1544) was a German physician and botanist who authored one of the greatest pharmacopoeias and one of the most celebrated herbals in history. He is also widely credited with developing a method for synthesizing ether (which he called by the poetic Latin name oleum dulci vitrioli, or "sweet oil of vitriol"). Cordus wrote prolifically, and identified and described several new plant species and varieties. The plant genus Cordia (紫草科破布木属)is named for him. Cordus’s Dispensatorium was soon followed by other pharmacopoeia in other parts of Germany and other countries of Europe. The first such book in the United States, the Lititz Pharmacopoeia, was published by Dr. William Brown in 1778 for use in military hospitals during the American Revolution. As in the modern world, plant materials were used not only to treat disease but also for other purposes, the most important of which was to produce hallucinogenic, psychedelic, or other “out-of-body” experiences. Many cultures throughout history have made the use of such materials an integral part of their religious ceremonies. For One of the most widely used of all illegal drugs is marijuana, which comes from the cannabis sativa plant. (Ted Kinsman/Photo Researchers, Inc.) example, ancient Hindu documents assert that the hallucinogenic effects of marijuana were fi rst discovered by the god Shiva, who ate leaves of the plant and found them very refreshing. Thereafter, the plant was routinely used in many Hindu ceremonies, usually in a form known as bhang. In the New World, the peyote cactus has been used in religious ceremonies for at least 10,000 years

Mescaline,or 3,4.5-trimethoxyphenethylamine,is a naturally ocuring pychedelic alkaloid of the phenethylamine cass.o ucinogenic effects comparable to those of LSD and psilocybin. It occurs naturally in the peyote cactus(Lophophor) the San Pedro cactus (Echinopsis pachanoi)the Peruvian torch (Echinopsis peruviana)and in a number of other members of the Cactaceae plant family.It is also found in small amounts in erain members of the Fabaceae (bean)family,including The cactus ()contains at least 40 chemicals with mind-altering properties the most important of which is mescaline(麦司卡林学名三甲氧苯乙胺,是苯乙胺的衍生物, 服用2-3小时后出现幻觉).Plant materials have also been used as drugs in some cultures for the killing of prey or in weapons used in battle.Perhaps the best known example of such use is the practice among some South American tribes of using curare,an extract of the plant Chondrodendron tomentosum.as a poison for the tips of their arrows used both in hunting and in warfare.The active ingredient in curare is the chemical known as D-tubocurarine Other South American tribes use a poison obtained from a group of amphibians known as poison dart frogs.These frogs are members of the family Dendrobatidae and belong primarily to the genera Dendrobates.Phyllobates,and Epipedobates.The most toxic member of the group is a frog known as Phyllobates terribilis,whose secretions are so toxic that they can cause serious illness to a human simply through contact with the skin.The most important active ingredient in the poison excreted by poison dart frogs is a chemical known as pumiliotoxin(两柄动物毒)
Mescaline, or 3,4,5-trimethoxyphenethylamine, is a naturally occurring psychedelic alkaloid of the phenethylamine class, known for its hallucinogenic effects comparable to those of LSD and psilocybin. It occurs naturally in the peyote cactus (Lophophora williamsii),[1] the San Pedro cactus (Echinopsis pachanoi),[2] the Peruvian torch (Echinopsis peruviana),[3] and in a number of other members of the Cactaceae plant family. It is also found in small amounts in certain members of the Fabaceae (bean) family, including Acacia berlandieri. [4] The cactus (仙人掌) contains at least 40 chemicals with mind-altering properties, the most important of which is mescaline (麦司卡林学名三甲氧苯乙胺,是苯乙胺的衍生物。 服用2-3小时后出现幻觉). Plant materials have also been used as drugs in some cultures for the killing of prey or in weapons used in battle. Perhaps the best known example of such use is the practice among some South American tribes of using curare, an extract of the plant Chondrodendron tomentosum, as a poison for the tips of their arrows used both in hunting and in warfare. The active ingredient in curare is the chemical known as D-tubocurarine. Other South American tribes use a poison obtained from a group of amphibians known as poison dart frogs. These frogs are members of the family Dendrobatidae and belong primarily to the genera Dendrobates, Phyllobates, and Epipedobates. The most toxic member of the group is a frog known as Phyllobates terribilis, whose secretions are so toxic that they can cause serious illness to a human simply through contact with the skin. The most important active ingredient in the poison excreted by poison dart frogs is a chemical known as pumiliotoxin (两栖动物毒素)

HO 4有机化学》.2004.24(10):1151-1158 W Daly, Gusovsky,ET Meneal,S Secunda,M Bell,Pumiliotoxin akaloids:a new class of sodium channel agents.Biochemical Pharmacology,1990,40(2):315-320 Natural Products and the Rise of Modern Chemistry Prior to the 19th century,practitioners of the healing arts knew essentially nothing about either the chemical composition of natural products or the mechanisms by which they work.They relied entirely on tradition,and trial and error,in the choices they made of the substances they used in their work.That situation began to change in the early 1800s with the rise of organic chemistry.Researchers began tofi nd ways to separate traditional drugs and medicines into their component parts, determine the chemicals of which they were made,elucidate their chemical structures, and,to some extent,synthesize the compounds in the laboratories It was a daunting task.In the vast majority of cases,the natural products traditionally used by healers are complex mixtures of dozens of chemical compounds. som f which may have medicinal properties,andsomfwhich may not.At first. the most that chemists could hope to accomplish was to obtain one or more active ingredients of plant materials in a pure form.To determine the chemical structures of these ingredients was,at the time,far beyond their capacity.Indeed,the molecular structures of chemicals obtained in a pure form in the early 1800s were often not determined until more than a hundred years later For example,one of the first chemicals to be purified from a natural product for use as a drug was morphine.In 1805.the German chemist Friedrich Wilhelm Sertumer(1783-1841)isolated the compound from opium while trying to find out how that substance induces sleep.He obtained morphine in a pure form,as white
(R) (R) (S) N (R) (E) (R) O (E) HO HO 《有机化学》, 2004, 24(10):1151-1158 JW Daly,F Gusovsky,ET Mcneal,S Secunda,M Bell,... Pumiliotoxin alkaloids: a new class of sodium channel agents. Biochemical Pharmacology, 1990, 40(2):315-326. Natural Products and the Rise of Modern Chemistry Prior to the 19th century, practitioners of the healing arts knew essentially nothing about either the chemical composition of natural products or the mechanisms by which they work. They relied entirely on tradition, and trial and error, in the choices they made of the substances they used in their work. That situation began to change in the early 1800s with the rise of organic chemistry. Researchers began to fi nd ways to separate traditional drugs and medicines into their component parts, determine the chemicals of which they were made, elucidate their chemical structures, and, to some extent, synthesize the compounds in the laboratories. It was a daunting task. In the vast majority of cases, the natural products traditionally used by healers are complex mixtures of dozens of chemical compounds, some of which may have medicinal properties, and some of which may not. At first, the most that chemists could hope to accomplish was to obtain one or more active ingredients of plant materials in a pure form. To determine the chemical structures of these ingredients was, at the time, far beyond their capacity. Indeed, the molecular structures of chemicals obtained in a pure form in the early 1800s were often not determined until more than a hundred years later. For example, one of the first chemicals to be purified from a natural product for use as a drug was morphine. In 1805, the German chemist Friedrich Wilhelm Sertürner (1783–1841) isolated the compound from opium while trying to find out how that substance induces sleep. He obtained morphine in a pure form, as white

crystals,but had no idea as to its chemical composition.That limitation did not prevent morphine's being put to use as a drug,however.In 1826,Emanuel Merck (1794-1855),founder of the great Merck Chemical company,began producing pure morphine commercially for use as a drug Still,the compound's chemical structure remained a mystery for more than a century.Finally,in 195,the English chemist Sir Robert Robinson determined the structural formula for morphine(with the exception of one uncertain atom). Another historically significant example is the story of quinine.For centuries. quinine was the most effective drug for the treatment of malaria,which has long been one of the world's most serious and widespread infectious diseases.The substance was first used as an antimalarial treatment in the 1600s,although how it was discovered it still not known with certainty.In any case,its importance as a drug inspired the search for methods of extracting it from its natural source and determining its chemical structure so that it could be made synthetically The first problem was solved in 1820 when the French chemist Pierre-Joseph Pelletier (1788-1842)and his associate Joseph-Bienaime Caventou found a way to extract quinine from cinchona bark.That accomplishment gave no clue,however,as to the compound's chemical structure,so chemists were unable to synthesize quinine analogs in the laboratory.(In pharmacology,an analog is a drug whose chemical structure is similar to that of another drug,but whose chemical and biological properties may be quite different.) Indeed,it was not until the 1920s that progress was made in that direction.Then researchers discovered that a number of compounds belonging to the aminoquinoline family were effective in the treatment of malaria.Between the 1920s and the 1950s these two compounds were the most effective antimalarials available.Still,the search for the chemical structure of quinine itself went on,a pursuit that was not successful until 1944.In that year,the American research team of Robert Burns Woodward (1917-79)and William von Eggers (1917-)completed the monumental task of elucidating the structure of qunine.With this knowledge,it became possible for chemists to begin producing quinine synthetically in the laboratory and,more
crystals, but had no idea as to its chemical composition. That limitation did not prevent morphine’s being put to use as a drug, however. In 1826, Emanuel Merck (1794–1855), founder of the great Merck Chemical company, began producing pure morphine commercially for use as a drug. Still, the compound’s chemical structure remained a mystery for more than a century. Finally, in 1925, the English chemist Sir Robert Robinson determined the structural formula for morphine (with the exception of one uncertain atom). Another historically significant example is the story of quinine. For centuries, quinine was the most effective drug for the treatment of malaria, which has long been one of the world’s most serious and widespread infectious diseases. The substance was first used as an antimalarial treatment in the 1600s, although how it was discovered it still not known with certainty. In any case, its importance as a drug inspired the search for methods of extracting it from its natural source and determining its chemical structure so that it could be made synthetically. The first problem was solved in 1820 when the French chemist Pierre-Joseph Pelletier (1788–1842) and his associate Joseph-Bienaimé Caventou found a way to extract quinine from cinchona bark. That accomplishment gave no clue, however, as to the compound’s chemical structure, so chemists were unable to synthesize quinine analogs in the laboratory. (In pharmacology, an analog is a drug whose chemical structure is similar to that of another drug, but whose chemical and biological properties may be quite different.) Indeed, it was not until the 1920s that progress was made in that direction. Then researchers discovered that a number of compounds belonging to the aminoquinoline family were effective in the treatment of malaria. Between the 1920s and the 1950s, these two compounds were the most effective antimalarials available. Still, the search for the chemical structure of quinine itself went on, a pursuit that was not successful until 1944. In that year, the American research team of Robert Burns Woodward (1917–79) and William von Eggers (1917– ) completed the monumental task of elucidating the structure of quinine. With this knowledge, it became possible for chemists to begin producing quinine synthetically in the laboratory and, more

important,to develop analogs that were even more effective than the natural product. The most effective of the quinine analogs was mefloquine,developed during the Vietnam War as the result of a program developed by the Walter Reed Army Institute for Research to protect American soldiers against malaria. The morphine and quinine stories have established a model for the study of natural products that has been repeated many times in recent history,that is.the search for the chemical structure of a biologically active substance so that(1)the compound can then be made synthetically and (2)analogs of the drug can be produced and tested for biological activity.(The term biological refers to the benefi cial or adverse effects of a drug on living materials.)One of the most exciting achievements in this type of research involved the study of a natural product that has been used by Chinese herbalists for thousands of years to treat fever.Called qing hoa,it is also known as sweet wormwood,annual wormwood,and sweet annie.Its systematic name is Artemisia an.In addition to its use as an antipyretic(antifever medication),qing hoa has been used effectively as an antimalarial drug Beginning in the 1960s,the People's Republic of China initiated an aggressive program to discover the scientifi c basis for many traditional herbal remedies. including qing hoa As a result of that program,in 1972 Chinese scientists identifi ed the active ingredient in qing hao,a substance they called qinghaosu.Qinghaosu is also known as arteannuin in China and as artemisinine in the West.Artemisinine is a sesquiterpene,a class of naturally occurring compounds with the general formula C15H24.The sesquiterpenes are considered to be chemically derived from the basic compound,isoprene.Chinese researchers have developed a number of derivatives of artemisinine,including the compounds known as artemether,artesunate.arteether and artelinate,all highly effective in the prevention and treatment of malaria The structural formulas of artemisinine and its derivatives are shown in the following diagram.The formulas show the close structural relationship of the compounds.differing only in the shaded portion of the molecules
important, to develop analogs that were even more effective than the natural product. The most effective of the quinine analogs was mefloquine, developed during the Vietnam War as the result of a program developed by the Walter Reed Army Institute for Research to protect American soldiers against malaria. The morphine and quinine stories have established a model for the study of natural products that has been repeated many times in recent history, that is, the search for the chemical structure of a biologically active substance so that (1) the compound can then be made synthetically and (2) analogs of the drug can be produced and tested for biological activity. (The term biological activity refers to the benefi cial or adverse effects of a drug on living materials.) One of the most exciting achievements in this type of research involved the study of a natural product that has been used by Chinese herbalists for thousands of years to treat fever. Called qing hoa, it is also known as sweet wormwood, annual wormwood, and sweet annie. Its systematic name is Artemisia annua. In addition to its use as an antipyretic (antifever medication), qing hoa has been used effectively as an antimalarial drug. Beginning in the 1960s, the People’s Republic of China initiated an aggressive program to discover the scientifi c basis for many traditional herbal remedies, including qing hoa. As a result of that program, in 1972 Chinese scientists identifi ed the active ingredient in qing hao, a substance they called qinghaosu. Qinghaosu is also known as arteannuin in China and as artemisinine in the West. Artemisinine is a sesquiterpene, a class of naturally occurring compounds with the general formula C15H24. The sesquiterpenes are considered to be chemically derived from the basic compound, isoprene. Chinese researchers have developed a number of derivatives of artemisinine, including the compounds known as artemether, artesunate, arteether and artelinate, all highly effective in the prevention and treatment of malaria. The structural formulas of artemisinine and its derivatives are shown in the following diagram. The formulas show the close structural relationship of the compounds, differing only in the shaded portion of the molecules

The success in determining the chemical structure of qinghaosu is only one example of the accomplishments of chemists in attaining a better understanding of the relationship between the chemical structure of natural drugs and their pharmacological effects.Those accomplishments have formed the basis of a whole new phase of the pharmaceutical industry in which natural products and their derivatives provide an extensive source of new drugs Microorganisms as the Source of Drugs Plants remained essentially the sole source of natural product drugs until well into the 20th century.Then in 1928 the discovery of penicillin by the Scottish bacteriologist Sir Alexander Fleming(1881-1955)opened an entirely new area of research in the field of Chemical formulas for artemisinine and its derivatives anti-infective drugs.Quite by accident,Fleming discovered that a sample of staphylococcus bacteria that he had inadvertently left out had begun to die out in certain areas of the culture.He determined that the change had come about in places where mold had fallen into the culture.Fleming isolated the mold and identifi ed it as Penicillim om.He inferred that the mold produced some chemical with the ability to attack and kill bacteria,a chemical that he later isolated and named penicillin. Penicillin was ony the first of a newcategory of drugs that cameto be caled antibiotics (named by the Russian microbiologist Selman Waksman in 1941). Antibiotics were originally defined as chemical substances produced by microorganisms and able to inhibit the growth of or destroy bacteria and other microorganisms.It took more than a decade for the significance of Fleming's discovery to be appreciated and for penicillin to be adopted by the medical profession as a treatment for infectious diseases.Once scientists turned that corner,however, they discovered a flood of new antibiotics in a relatively short period of time streptomycin,by Waksman in 1943.bacitracin,by American bacteriologist Frank
The success in determining the chemical structure of qinghaosu is only one example of the accomplishments of chemists in attaining a better understanding of the relationship between the chemical structure of natural drugs and their pharmacological effects. Those accomplishments have formed the basis of a whole new phase of the pharmaceutical industry in which natural products and their derivatives provide an extensive source of new drugs. Microorganisms as the Source of Drugs Plants remained essentially the sole source of natural product drugs until well into the 20th century. Then in 1928 the discovery of penicillin by the Scottish bacteriologist Sir Alexander Fleming (1881–1955) opened an entirely new area of research in the field of Chemical formulas for artemisinine and its derivatives anti-infective drugs. Quite by accident, Fleming discovered that a sample of staphylococcus bacteria that he had inadvertently left out had begun to die out in certain areas of the culture. He determined that the change had come about in places where mold had fallen into the culture. Fleming isolated the mold and identifi ed it as Penicillium notatum. He inferred that the mold produced some chemical with the ability to attack and kill bacteria, a chemical that he later isolated and named penicillin. Penicillin was only the first of a new category of drugs that came to be called antibiotics (named by the Russian microbiologist Selman Waksman in 1941). Antibiotics were originally defined as chemical substances produced by microorganisms and able to inhibit the growth of or destroy bacteria and other microorganisms. It took more than a decade for the significance of Fleming’s discovery to be appreciated and for penicillin to be adopted by the medical profession as a treatment for infectious diseases. Once scientists turned that corner, however, they discovered a flood of new antibiotics in a relatively short period of time: streptomycin, by Waksman in 1943; bacitracin, by American bacteriologist Frank