
Chapter 6 Thermodynamic properties of fluids Why to study this chapter In industrial processes,we need: Thermodynamic properties (1)T.P.Cv.Co. ②UH.s.G. Can be measured How to do calculated thermodynamics 学习化工热力学的目的在于应用,主要应用之一就是热力学性质的推算, 从容易测量的性质一难测量的性质: 从基础物性→更多有用的性质: 从纯物质性质→混合物性质. 热力学原理+模型解决上述问题 从均相封闭体系经典热力学原理,得到不同的热力学性质之间的普遍化关系,特别是热 力学性质与P.VT之间的关系 结合一定的状态方程,这些关系式就成为计算特定的均相纯物质或均相定组成混合物性 质的公式 6 Thermodynamic properties of fluids 6.1 property Relations for Homogeneous Phase 6.2 Residual Properties 63 Residual properties by equations of State 6.4 Two-phase systems 6.5 Thermodynamic Diagrams 6.6 Tables of Thermodynamic Properties 6.7 Generalized Property Correlations for Gases 6.1 property Relations for Homogeneous Phase us Phase System 系式) 6.1.3 Maxwel's Equation(Maxwell关系式) 6.1.4 Application of Maxwell's Equation(Maxwell关系式的应用)
Chapter 6 Thermodynamic properties of fluids Why to study this chapter ? In industrial processes, we need: 学习化工热力学的目的在于应用,主要应用之一就是热力学性质的推算。 热力学原理+模型解决上述问题 从均相封闭体系经典热力学原理,得到不同的热力学性质之间的普遍化关系,特别是热 力学性质与 P-V-T 之间的关系 结合一定的状态方程,这些关系式就成为计算特定的均相纯物质或均相定组成混合物性 质的公式 6 Thermodynamic properties of fluids 6.1 property Relations for Homogeneous Phase 6.2 Residual Properties 6.3 Residual Properties by Equations of State 6.4 Two-phase systems 6.5 Thermodynamic Diagrams 6.6 Tables of Thermodynamic Properties 6.7 Generalized Property Correlations for Gases 6.1 property Relations for Homogeneous Phase 6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System 6.1.2 The Criterion of Exactness for a Differential Expression(恰当微分准则,点函数间的数学关 系式) 6.1.3 Maxwell’s Equation (Maxwell 关系式) 6.1.4 Application of Maxwell’s Equation (Maxwell 关系式的应用) Thermodynamic properties How to do ? (1) T, P, Cv, Cp, . (2) U, H, S, G, . Can be measured Need to be calculated Equations of state Fundamental property relations of thermodynamics + 从容易测量的性质→难测量的性质; 从基础物性→更多有用的性质; 从纯物质性质→混合物性质
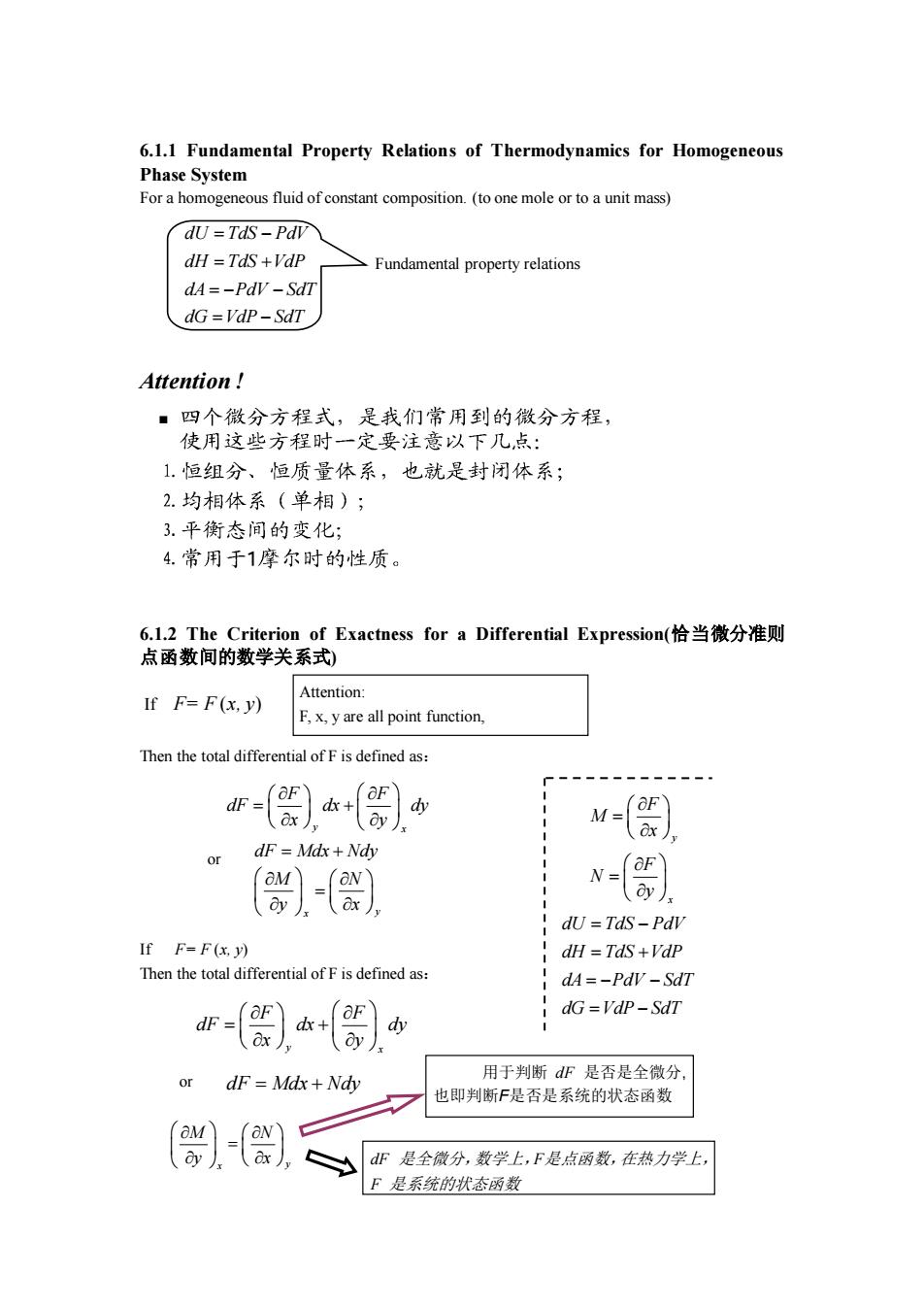
6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System For a homogeneous fluid of constant composition.(to one mole or to a unit mass) dU=Tds-Pdv dH =TdS +VdP Fundamental property relations dA=-PdV-SdT dG=VdP-SdT Attention! 1恒组分、恒质量体系,也就是封闭体系; 2.均相体系(单相); 3.平衡态间的变化: 4.常用于1摩尔时的性质 6.l.2 The Criterion of Exactness for a Differential Expression(恰当微分准则 点函数间的数学关系式) If F=F(x.y) Attention F.x.yare all point function. Then the total differential ofF is defined as: or dF=Md体+N v-) dU=TdS-Pdl If F-F(x.y) dH TdS+Vdp Then the total differential of F is defined as: dA=-PdV-SdT dG=VdP-SdT or dF Mdx+Ndhy 用于判断dF是否是全微分, 也即判断F是否是系统的状态函数 dF是全微分,数学上,F是点函数,在热力学上, F是系统的状态丽数
6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System For a homogeneous fluid of constant composition. (to one mole or to a unit mass) Attention ! 6.1.2 The Criterion of Exactness for a Differential Expression(恰当微分准则 点函数间的数学关系式) Then the total differential of F is defined as: If F= F (x, y) Then the total differential of F is defined as: If F= F (x, y) Attention: F, x, y are all point function, and F is the continuous function about x, y Fundamental property relations dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − y x F M x F N y = = dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − y x F F dF dx dy x y = + dF Mdx Ndy = + x y M N y x = or y x F F dF dx dy x y = + or dF Mdx Ndy = + x y M N y x = dF 是全微分,数学上,F是点函数,在热力学上, F 是系统的状态函数 用于判断 dF 是否是全微分, 也即判断F是否是系统的状态函数
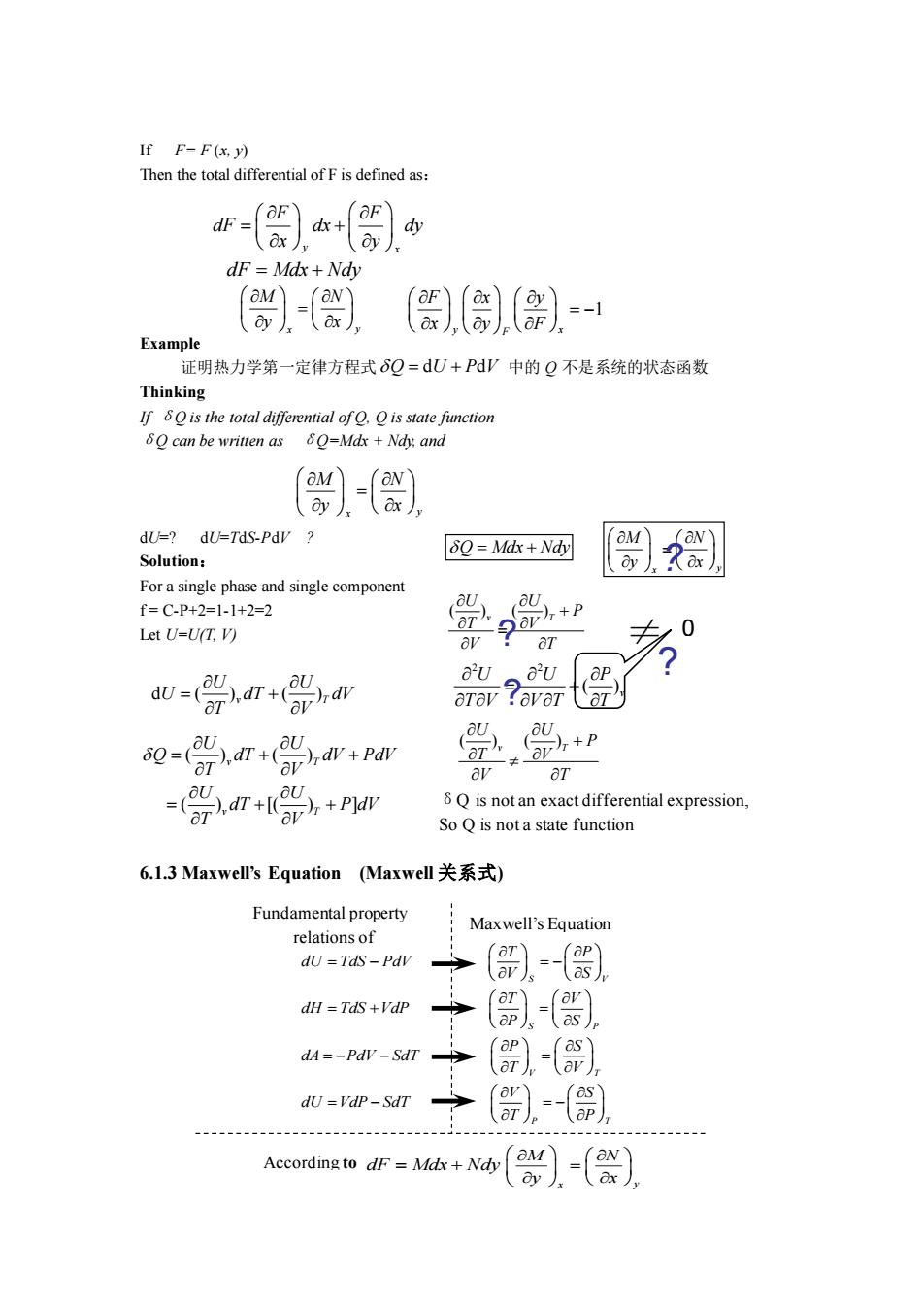
If F=F(x.y) dF Mdx +Ndy )〔- Exampl 证明热力学第一定律方程式8Q=dU+PΨ中的Q不是系统的状态函数 Thinking Q can be written as -劉 dU=?dU=TdS-PdV Solution: ⊙Q=M体+ For a single phase and single component f=C-P+2=1-1+2=2 Let U-U(T:V) p岛 aΨ 0 dv=( arev ?avara 0-n+0w+m =n++Pr 8Q is not an exact differential expression, So Q is nota state function 6.l.3 Maxwell's Equation(Maxwell关系式) Fundamental property Maxwell's Equation (部)-() (-(器), dA=-PdV-SdT ()-). dU=VdP-SdT ,- According to dF=Mdx+Ndy ()-(器
If F= F (x, y) Then the total differential of F is defined as: Example 证明热力学第一定律方程式 中的 Q 不是系统的状态函数 Thinking If δQ is the total differential of Q, Q is state function δQ can be written as δQ=Mdx + Ndy, and dU=? dU=TdS-PdV ? Solution: For a single phase and single component f = C-P+2=1-1+2=2 Let U=U(T, V) 6.1.3 Maxwell’s Equation (Maxwell 关系式) y x F F dF dx dy x y = + dF Mdx Ndy = + x y M N y x = 1 y x F F x y x y F = − Q U P V = + d d x y M N y x = d ( ) ( ) v T U U U dT dV T V = + ( ) ( ) ( ) [( ) ] v T v T U U Q dT dV PdV T V U U dT P dV T V = + + = + + Q Mdx Ndy = + x y M N y x = ? ( ) ( ) v T U U P T V V T + = 2 2 ( )v U U P T V V T T = + 0 ? ≠ ? ? ( ) ( ) v T U U P T V V T + δQ is not an exact differential expression, So Q is not a state function Maxwell’s Equation Fundamental property relations of Thermodynamics x y M N y x = According to dF Mdx Ndy = + V S P T V T S P P S dA PdV S V T V dH TdS VdP S dU VdP Sd P T P dU TdS dT PdV V T T P S T SV = − = − = + = = − = − = − − =

Thinking(6-1) =,=T dU Tds-Pdv Obtain these formula from dH TdS +Vdp dA=-PdV-Sal dG=VdP-SdT For example V=Constant dU =TdS-Pdv dU =Tds-Pdv as )(oH dH =Tds+Vdp dH =Tds+Vdp dA=-Pdv-SdT dA=-PdV-SdT dG=VdP-SdT dG=VdP-SdT P=Constant 解:思路 xar OT-ovior) o (ov /aP) Volume expansivity -0018 k=- 号-g008-46MmIK 4.675×(277-275)=9.35MPa Isothermal compressibility Thinking(6-2) 满瓶液氯压力与温度的关系 温度℃05 101520253035 瓶压kcm22.771131184240275325356 Thinking(6-3) 1.热力学基本关系式dH=TdS+'dP只适用于可逆过程 (错。不需要可逆条件,适用于只有体积功存在的封闭体系 2.象dU-TdS-Pdl等热力学基本方程只能用于气体,而不能用于液体或固相。 (错。能田于任向何相态) 3.对于一均匀的物质,其H和U的关系为 A HU CH=UD不能确定 (B。因H=U+PV) 4.某人声明所建立的纯固体的状态方程和热力学能的方程分别为
Thinking(6-1) For example Example 试计算在 0.01013MPa 下,液态汞由 275K 恒容加热到 277K 时产生的压力 解:思路 Thinking(6-2) 满瓶液氯压力与温度的关系 温度℃ 0 5 10 15 20 25 30 35 瓶压 kg/cm2 2.7 71 131 184 240 275 325 356 Thinking(6-3) 1. 热力学基本关系式 dH=TdS+VdP 只适用于可逆过程。 (错。不需要可逆条件,适用于只有体积功存在的封闭体系) 2. 象 dU=TdS-PdV 等热力学基本方程只能用于气体,而不能用于液体或固相。 (错。能用于任何相态) 3. 对于一均匀的物质,其 H 和 U 的关系为 A HU C H=U D 不能确定 (B。因 H=U+PV) 4.某人声明所建立的纯固体的状态方程和热力学能的方程分别为 ( ) ( ) V P U H T S S = = dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − P=Constant V=Constant dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − ( ) ( ) ( ) ( ) ( ) ( ( ) ( ) ) S T S T P V V P U A P V V H G V P P G A S T H T T U S S = = − = = = = − = = Obtain these formula from P T T ( )V ( / ) ( / ) P T P V T T V P = − ( )V 1 0.00018 V K T − = P 1 = ( ) V 1 0.0000385 V K P − = T 1 k=- ( ) V 0.00018 4.675 / 0.0000385 P MPa K T = = ( )V 4.675 (277 275) 9.35 − = MPa Volume expansivity Isothermal compressibility
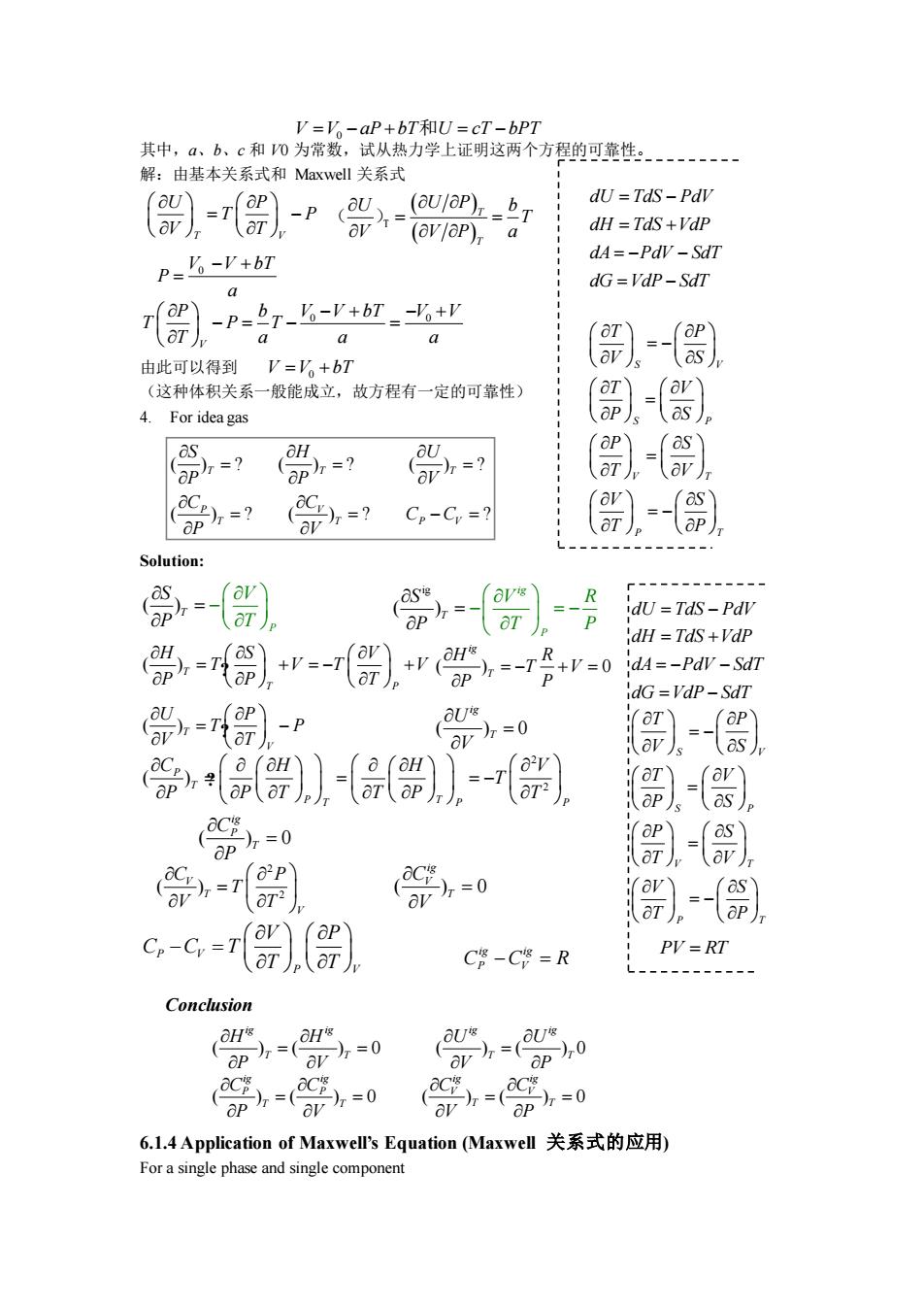
v-V .aD+hT和IJ=T-hpT 其中,、b、c和0为常数,试从热力学上证明这两个方程的可靠性. 解:由基本关系式和Mawd关系式 ()-r-p0- dU TdS-PdV (av/op)a dH =TdS+VdP P=6-P+b7 dA=-PdV-SdT a dG=VdP-SdT )-p-rΨ a 由此可以得到V=V。+bT ()=-s) (这种体积关系一般能成立,故方程有一定的可靠性) 4.For ideagas - ()- =?=?G-G= Solution 常 dU Tds-Pdv dH=TdS+VdP 0-)层r dA=-PdV-SdT dG =Vdp-SdT 0=r)-p U ar)=0 0 -s) ap 0 G-c-).() C-C=R PV=RT Conclusion =0 等=0 6.L.4 Application of Maxwell's Equation(Maxwel关系式的应用) For a single phase and single component
其中,a、b、c 和 V0 为常数,试从热力学上证明这两个方程的可靠性。 解:由基本关系式和 Maxwell 关系式 由此可以得到 (这种体积关系一般能成立,故方程有一定的可靠性) 4. For idea gas Solution: 6.1.4 Application of Maxwell’s Equation (Maxwell 关系式的应用) For a single phase and single component T V U P T P V T = − ( ) ( ) T T U b U P T V V P a = = ( )T V V bT 0 P a − + = 0 0 V P b V V bT V V T P T T a a a − + − + − = − = ig ( ) ig T P R P T P S V − = − = 2 2 ( ) P T T T P P P H H V T P P T T P T C = = − = ( ) T P T S V T V T V P H P T + = − + = ? ? ( ) 0 ig T H P R T V P = − + = 2 2 ( ) V T V C P T V T = ( ) 0 ig P T C P = ( ) 0 ig V T C V = ( )T V P T P U V T − = ? ( ) 0 ig T U V = P V P V V P C C T T T − = ig ig C C R P V − = ( ) P T V T S P − = V V aP bT U cT bPT = − + = − 0 和 S P T V T S P V P S V S T P T V P SV P V S T T = − = = − = dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − V V bT = +0 ( ) ? ( ) ? ( ) ? ( ) ? ( ) ? ? T T T P V T T P V S H U P P V C C C C P V = = = = = − = S P T V T S P V P S V S T P T V P SV P V S T T = − = = − = dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − PV RT = Conclusion ( ) ( ) 0 ig ig T T H H P V = = ( ) ( ) 0 ig ig T T U U V P = ( ) ( ) 0 ig ig P P T T C C P V = = ( ) ( ) 0 ig ig V V T T C C V P = =
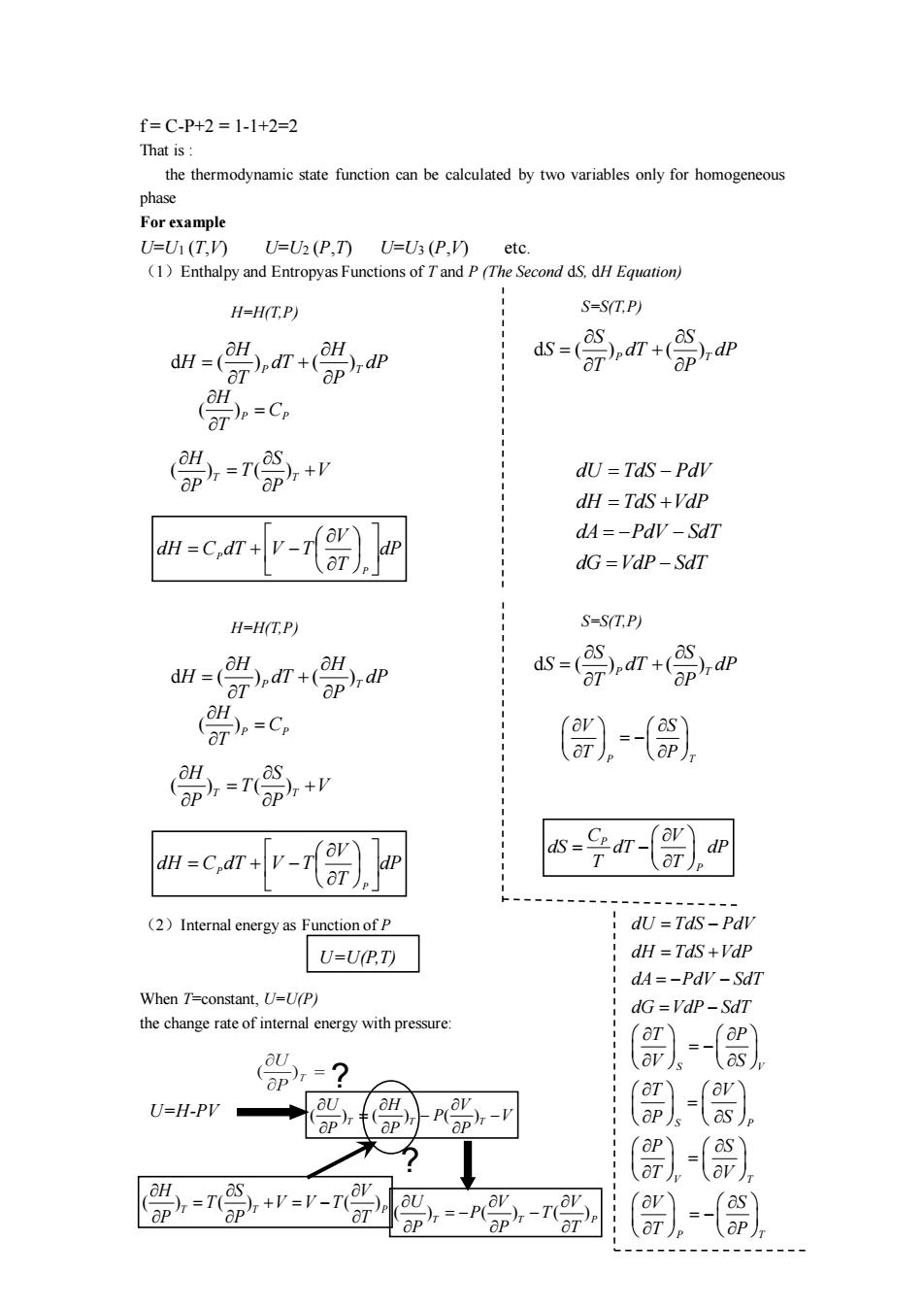
f=C-P+2=1-1+2=2 That is the thermodynamic state function can be calculated by two variables only for homogeneous phase For example U=U(T.V) UU(P n UU(P (1 Enthalpy ad Entropyas Functions Second H-H(T.P) S=S(T.P) d=欲n+0n (.c 0=n+w dU =TdS-Pdv dH TdS+VdP m-c.mv-ror) dA=-PdV-SdT dG=VdP-SdT H-H(T.P) S=S(T.P) n (,dr(),dp dS-(dp 0=C, , 0-n+w w.civ-) (2)Internal energy as Function of P dU=TdS-Pdl U=UP.T) dH =TdS+Vdp dA=-PdV-Sdl When T=constant,U-U(P) dG=VdP-SdT the change rate of interal energy with pressure 02 U=H-PV -】 -) .r-n P=-P-7
f = C-P+2 = 1-1+2=2 That is : the thermodynamic state function can be calculated by two variables only for homogeneous phase For example U=U1 (T,V) U=U2 (P,T) U=U3 (P,V) etc. (1)Enthalpy and Entropyas Functions of T and P (The Second dS, dH Equation) (2)Internal energy as Function of P When T=constant, U=U(P) the change rate of internal energy with pressure: H=H(T,P) S=S(T,P) d ( ) ( ) P T H H H dT dP T P = + d ( ) ( ) P T S S S dT dP T P = + ( )P P H C T = ( ) ( ) T T H S T V P P = + dP T V dH C dT V T P P = + − dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − H=H(T,P) S=S(T,P) d ( ) ( ) P T H H H dT dP T P = + d ( ) ( ) P T S S S dT dP T P = + ( )P P H C T = ( ) ( ) T T H S T V P P = + dP T V dH C dT V T P P = + − P T V S T P = − P P C V dS dT dP T T = − U=U(P,T) dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − S P T V T S P V P S V S T P T V P SV P V S T T = − = = − = ( ) ( ) ( ) T T P H S V T V V T P P T = + = − ( ) ( ) ( ) T T P U V V P T P P T = − − ? U=H-PV ? ( ) ( ) ( ) T T T U H V P V P P P = − −
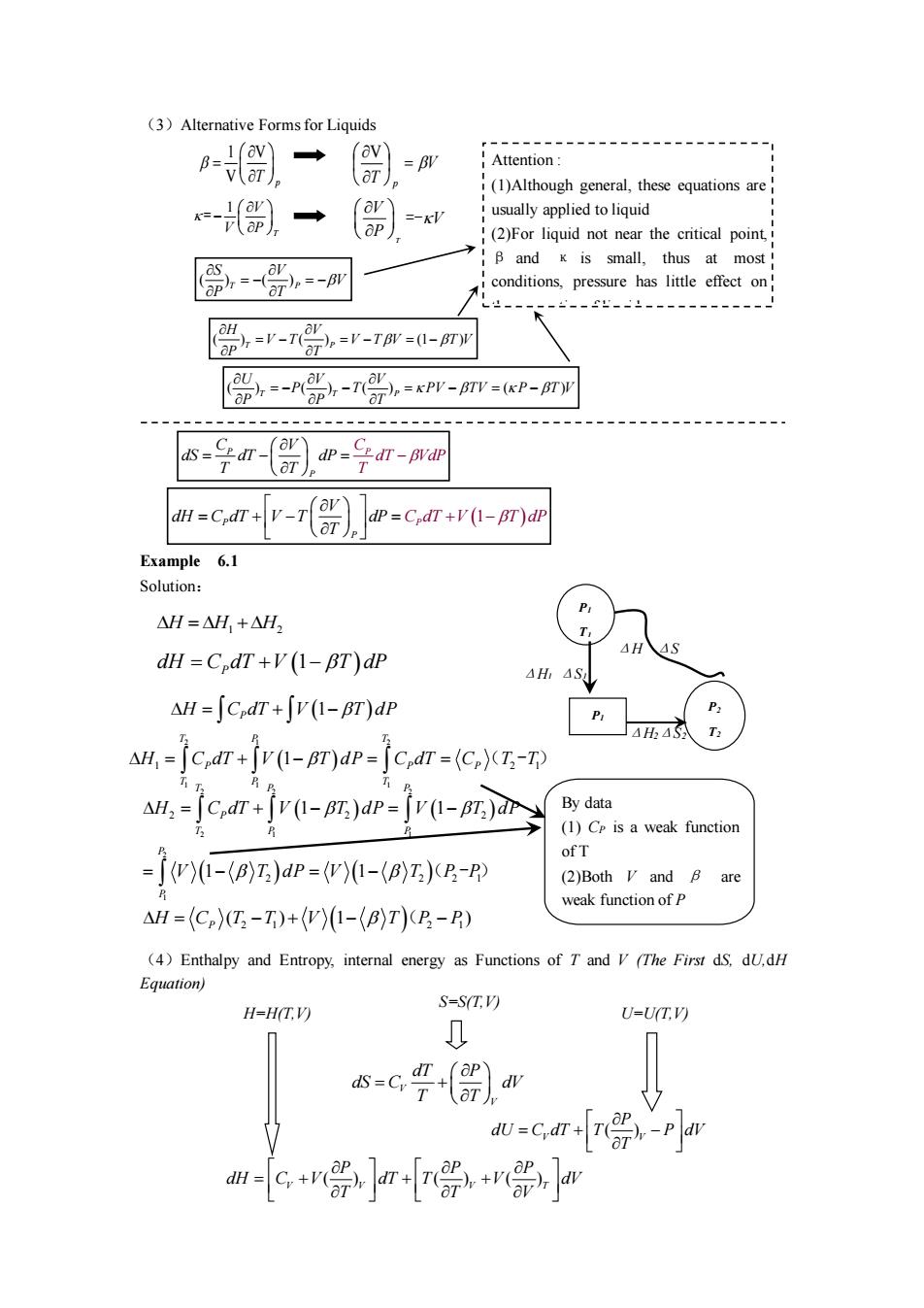
(3)Alternative Forms for Liquids (av) =BV Attention (1)Although general,these equations are -别一 usually applied to liquid (2)For liquid not near the critical point. B and x is small.thus at most 党 conditions.pressure has little effect on =-=-Tm=-m =-A0-0=Pr-mr=P-m 号r-,即号r-m dP=C,dT+v(1-BT)d Example 6.1 Solution: △H=H,+△H, dH=C dT+V(1-BT)dP AH,AS △H=∫cdr+jv(I-BT)dr 4h4 AH,=[C,dT+v(1-BT)dP=[C,dT=(C)(T-T) AH,-jc,d+jv(1-BT)adp=fv(-BT)r By data (1)C is a weak function -了y-(Bz)dP=w-z)水R-P (2)Both V and 6 are △H=(C)I,-T)+(W)1-(E)T)B-R) weak function ofp ()Enthalpy and Entropy,internal energy as Functions of T and V(The First dS.d.dH Equation) H=H(TV) S=S(T.V) U=U(T.V) s-c() =cr+r号pr m-crw
(3)Alternative Forms for Liquids Example 6.1 Solution: (4)Enthalpy and Entropy, internal energy as Functions of T and V (The First dS, dU,dH Equation) ( ) ( ) T P V P T V S − = = − ( ) ( ) (1 ) T P H V V T V T V P T T V = − = − = − ( ) ) ( ) ( ( ) T T P U V V P T PV TV P T V P P T = − − = − = − Attention : (1)Although general, these equations are usually applied to liquid (2)For liquid not near the critical point, β and κ is small, thus at most conditions, pressure has little effect on the properties of liquid 1 V V T p = V T p = V 1 T V V P − = T V V P =- P P P C dT V C V dS dT dP T T dP T = − = − P (1 ) P P V dH C dT V T dP T C dT V T dP = + − = + − P1 T1 H1 S1 P2 T2 H2 S2 ΔH ΔS P1 T2 ΔH2ΔS2 ΔH1 ΔS1 = + H H H 1 2 dH = C dT T P P + − V (1 ) d = + − H C dT V T dP P (1 ) ( ) 2 1 1 1 2 1 1 1 2 1 T P P T P T P T = − H C dT V T d + P = C dT = CP T T ( - ) ( ) ( ) ( ) ( ) 2 1 2 2 2 2 1 1 2 2 2 2 2 2 1 1 1 1 1 T P P P P T P P P V T dP V H C T dP V T P P dT V T dP = + = − = − − = − ( - ) = − + − − H C T T V T P P P ( ) 1 ) 2 1 2 1 ( () By data (1) CP is a weak function of T (2)Both V and β are weak function of P H=H(T,V) U=U(T,V) S=S(T,V) V V dT P dS C dV T T = + ( ) ( ) ( ) V V V T P P P dH C V dT T V dV T T V = + + + ( ) V V P dU C dT T P dV T = + −

Thinking(6-4) 1.一气体符合P=RTb)的状态方程从门等温可逆膨胀至2,则体系的S为 DRn片 (c) 2对于一均相体系》,-》等于 B Ce/Cv CR D )(). (D) 》.-)-c-c-.(】 (6)The Gibbs Energy as a Generating Function dG=VdP-SdT au=ids-pay- dH =TdS+VdP 1 G RT 2、 dG=VdP-SdT U=H-PV d是r= H G=H-TS A=U-TS Applied in restricted form T=constan RT-GIRT) ap p-constant H -n0G1RD1。 RT T The Gibbs energy when given as a function of T and P therefore serves as a generating function for the other thermodynamic properties,and impicitly represents complete property information 6.2 Residual Properties(偏离函数剩余性质) 6.2.1 Residual Property Where Mis the molar value of any extensive thermodynamic property,eg.U,H.S.HorG. M and Mig,the actual and ideal-gas properties,are at the same temperature and pressur 在相同的温度和压力下,真实气体的热力学性质与型想气体的蕊力学丝厦的差值。(其中 M代表U、H、S、G等)
Thinking(6-4) 1. 一气体符合 P=RT/(V-b)的状态方程从 V1 等温可逆膨胀至 V2,则体系的 S 为 2. 对于一均相体系 等于 (6)The Gibbs Energy as a Generating Function Applied in restricted form The Gibbs energy when given as a function of T and P therefore serves as a generating function for the other thermodynamic properties, and implicitly represents complete property information 6.2 Residual Properties(偏离函数 剩余性质) 6.2.1 Residual Property The definition for the generic residual property: M R≡M-Mig Where M is the molar value of any extensive thermodynamic property, e.g., V, U, H, S, H or G. M and Mig, the actual and ideal-gas properties, are at the same temperature and pressure 在相同的温度和压力下,真实气体的热力学性质与理想气体的热力学性质的差值。(其中 M 代表 U、H、S、G 等) ( / ) [ ]T V G RT RT P = ( / ) [ ]P H G RT T RT T = − T=constan t p=constant V P P V T T T A 0 B CP/CV C R D 2 1 ln V b RT V b − − 2 1 ln V b R V b − − 2 1 ln V R V A B 0 C D 2 2 2 1 1 1 2 1 ln V V V V V V T V S P R V b S dV dV dV R V T V b V b − = = = = − − (C) P V S S T T T T − P V P V V P S S P V T T C C T T T T T − = − = (D) dG VdP SdT = − 2 1 1 ) 1 ( ) ( G d dG Gd RT RT RT G dG dT RT RT = + = − 2 2 1 ( ) ( ) G H TS d VdP SdT dT RT RT R V H dP dT T RT RT − = − − = − dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − G H TS A U V S U P T H = − = − = −
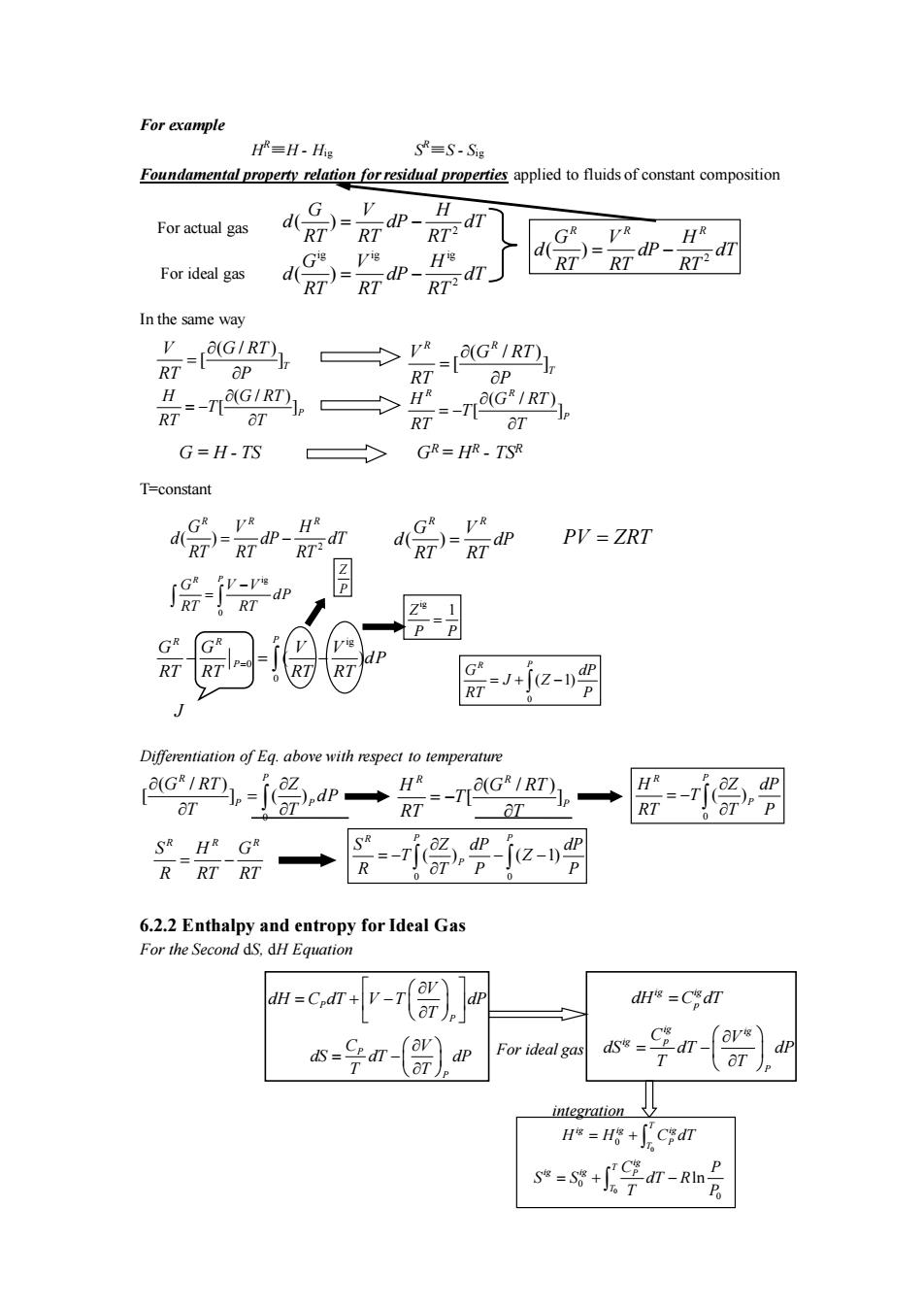
For example H=H-His S=S-Sg Foundamental property relation forresidal properties appliedto fuids of constant composition H For actual gas G HR For ideal gas RT)-RTdp- In the same way -G'IRT), RT >所=-nG吗 aT G=H-TS GR=HR-TSR T-constant HR R RT)= PV=ZRT =-je-吗 Differentiation of Eq.above with respect to temperature RT ]p◆ aT SR HR GR R 层停2-周 6.2.2 Enthalpy and entropy for Ideal Gas For the Second dS.dH Equation -cv-r(r)un dH=CdT s=号r-()p For ideal go a-) integration H=Hg+∫Cgdr s*=g+号dr-m月
P For ideal gas P C V dS dT dP T T = − P P V dH C dT V T dP T = + − ig ig ig p P C V dS dT dP T T = − ig ig dH C dT = p 0 0 T ig ig ig P T H H C dT = + 0 0 0 ln ig T ig ig P T C P S S dT R T P = + − integration For example H R≡H - Hig S R≡S - Sig Foundamental property relation for residual properties applied to fluids of constant composition In the same way T=constant Differentiation of Eq. above with respect to temperature 6.2.2 Enthalpy and entropy for Ideal Gas For the Second dS, dH Equation 0 ( / ) [ ] ( ) R P P P G RT Z d P T T = ( / [ ]) R P R G RT T H T RT = − 0 0 ( ) ( 1) P R P P Z dP dP T Z T P S R P − − − = R R R S H G R RT RT = − 0 ( ) R P P Z P R T H T T d P − = ( ) R R G d P T V R d RT = 2 ( ) R R R G V H d dP dT RT RT RT = − ig 0 R P G V V dP RT RT − = PV ZRT = Z P ig Z 1 P P = 0 ( 1) R P G dP J Z RT P = + − ig 0 0 ( ) R R P P G G V V d P RT RT RT RT − = − = J ( / ) [ ] R R T V G RT RT P = ( / ) [ ] R R P H G RT T RT T = − ( / ) [ ]T V G RT RT P = ( / ) [ ]P H G RT T RT T = − G = H - TS GR = HR - TSR 2 ( ) G V H d dP dT RT RT RT = − ig ig ig 2 ( ) G V H d dP dT RT RT RT = − 2 ( ) R R R G V H d dP dT RT RT RT = − For ideal gas For actual gas

H=Hs+HR=HC路d+H H=H+H=H8+(C) T-T)+H* 5=+5-+GR ∫Cgdn (C〉,=互 ∫C$dr=R×ICPH(T0,T,A,B,C,D)∫Ctdr=R×CPST0,T,A,B,C,D) 6.2.3 Calculation for Residual Enthalpy and Entropy j停若-j停-j2-光 剩余焓和剩余熵的计算方法:①根据PVT实验数据计算②状态方程法③普遍化关系法 Thinking(6-5) 1当压力于零时 M(T,P)-M(T,P)=0 (M是摩尔性质) (错。当M=V时,不恒等于零) 2.由于偏离函数是两个等温状态的性质之差,故不可能用偏离函数来计算热力学性质随若温 府的弯化 M(T.P)-M(T.B) (销。因为: =[M(I,)-M(I,E)】-[M(T,P)-M(I,E)】]+[M(I,B)-M(T,E] 3.由一个优秀的状态方程,就可以计算所有的均相热力学性质随若状态的变化。 Example 6.3 (错。还需要C(T)模型) Solution: Clue: H=Hg+∫C:dr =g+孚n-Rn H=H+H S=SW+S S R require evaluation of the two integrals by graphical integration
6.2.3 Calculation for Residual Enthalpy and Entropy 剩余焓和剩余熵的计算方法:① 根据 P-V-T 实验数据计算② 状态方程法③ 普遍化关系法 Thinking(6-5) 1.当压力趋于零时 (错。当 M=V 时,不恒等于零) 2.由于偏离函数是两个等温状态的性质之差,故不可能用偏离函数来计算热力学性质随着温 度的变化。 3.由一个优秀的状态方程,就可以计算所有的均相热力学性质随着状态的变化。 Example 6.3 Solution: Clue: require evaluation of the two integrals by graphical integration ( , , 0 ) ( ) ig M T P M T P − (M是摩尔性质) 0 0 T ig R ig ig R P T H H H H C dT H = + = + + 0 0 0 ln ig T ig R ig R P T C P S S S S dT R S T P = + = + − + 0 ( ) ig R ig ig R P H H H H H C T T H = + = + − + 0 0 0 ln ln ig R ig ig R P S T P S S S S C R S T P = + = + − + 0 0 0 0 ln T T ig ig P P ig ig T T P P H S dT C dT C T C C T T T T = = − 0 0 ( 0, , , , , ) ( 0, , , , , ) T T ig ig P P T T C dT R ICPH T T A B C D C dT R ICPS T T A B C D = = 0 ( ) R P P Z P R T H T T d P − = 0 0 ( ) ( 1) P R P P Z dP dP T Z T P S R P − − − = (错。因为: ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) 2 2 1 1 2 2 2 0 1 1 1 0 2 0 1 0 , , , , , , , , ig ig ig ig M T P M T P M T P M T P M T P M T P M T P M T P − = − − − + − (错。还需要 ( ) 模型) ig C T P 0 ( ) R P P Z dP T T P H RT = − 0 0 ( ) ( 1) P R P P Z dP dP T Z T P S R P − − − = ig R H H H = + ig R S S S = + 0 0 T ig ig ig P T H H C dT = + 0 0 0 ln ig T ig ig P T C P S S dT R T P = + −