
New drugs development 新药研发 Professor Lingling Zhang(张玲玲) Institute of clinical pharmacology,AHMU 安徽医科大学临床药理研究所) Izhang@ahmu.edu.cn
New drugs development 新药研发 Professor Lingling Zhang (张玲玲 ) Institute of clinical pharmacology, AHMU (安徽医科大学临床药理研究所 ) llzhang@ahmu.edu.cn

Outlines I.Preclinical development of new drugs; II.Clinical trial of new drugs: 1.GCP and the significance of implementing GCP. 2.International Council for Harmonization,ICH 3.Phase I clinical trial tolerance test and pharmacokinetic studies. 4.Phase II clinical trial. 5.Phase III clinical trial. 6.Phase IV clinical trial. 7.Drug bioequivalence test. III.Drug consistency evaluation 2019/8/28 Institute of clinical pharmacology,AHMU 2
Outlines I. Preclinical development of new drugs; II. Clinical trial of new drugs: 1.GCP and the significance of implementing GCP. 2. International Council for Harmonization, ICH 3. PhaseⅠ clinical trial : tolerance test and pharmacokinetic studies. 2019/8/28 Institute of clinical pharmacology, AHMU 2 4. Phase II clinical trial. 5. Phase III clinical trial. 6. Phase IV clinical trial. 7. Drug bioequivalence test. III. Drug consistency evaluation

Aims 1.To know of: (1)The definition of drugs,new drug and bioavailability. (2)The components of preclinical drug development. 2.To master (1)The definition of GCP. (2)The determination of minimum first dose. (3)The purpose and significance Phase II clinical trial. (4)The purpose and significance of human bioavailability test. 2019/8/28 Institute of clinical pharmacology,AHMU 3
Aims 1. To know of : (1) The definition of drugs, new drug and bioavailability. (2) The components of preclinical drug development. 2. To master : (1) The definition of GCP . 2019/8/28 Institute of clinical pharmacology, AHMU 3 (2) The determination of minimum first dose. (3) The purpose and significance Phase II clinical trial. (4) The purpose and significance of human bioavailability test
A example: Headache Drug Alleviation Question:What's drug? 2019/8/28 Institute of clinical pharmacology,AHMU 4
A example: Headache Drug Alleviation 2019/8/28 Institute of clinical pharmacology, AHMU 4 Headache Drug Alleviation Question: What’s drug?

The definition of drugs and new drugs 1.Drugs:drugs are some materials,which are used for the prevention,treatment and diagnosis of diseases,regulating physiological function,and having indications,major functions and usage. 2019/8/28 Institute of clinical pharmacology,AHMU 5
The definition of drugs and new drugs 1.Drugs: drugs are some materials, which are used for the prevention, treatment and diagnosis of diseases, regulating physiological function, and having indications, major functions and usage. 2019/8/28 Institute of clinical pharmacology, AHMU 5

2.New drugs:new drugs are the drugs that are not posted in state pharmacopoeia()or approved to be used by the state department of pharmaceutical administration. (National Medical Products Administration,NMPA) 国家药品监督管理局 National Medical Products Administration 中华人民共和国 药典 通想 木神机由 +中4aa。 2019/8/28 Institute of clinical pharmacology,AHMU 6
2.New drugs: new drugs are the drugs that are not posted in state pharmacopoeia(药典) or approved to be used by the state department of pharmaceutical administration. (National Medical Products Administration, NMPA) 2019/8/28 Institute of clinical pharmacology, AHMU 6

I Preclinical development of new drugs 2019/8/28 Institute of clinical pharmacology,AHMU
I Preclinical development of new drugs 2019/8/28 Institute of clinical pharmacology, AHMU 7
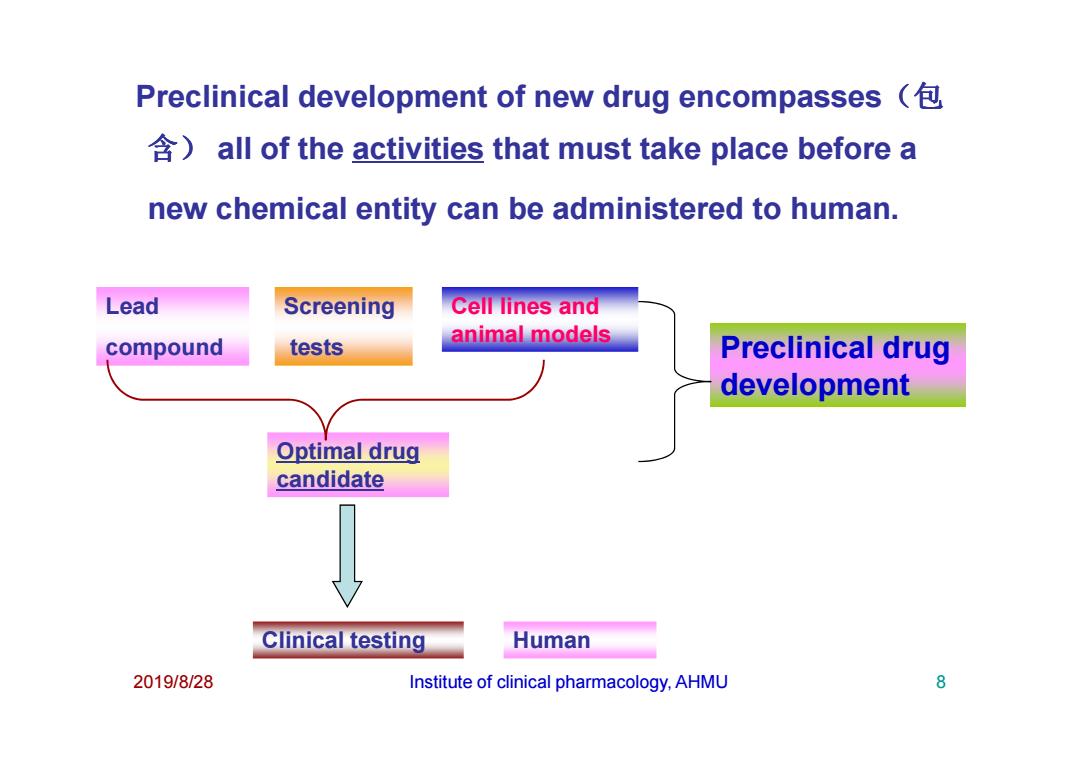
Preclinical development of new drug encompasses all of the activities that must take place before a new chemical entity can be administered to human. Lead Screening Cell lines and compound tests animal models Preclinical drug development Optimal drug candidate Clinical testing Human 2019/8/28 Institute of clinical pharmacology,AHMU 8
Lead compound Screening tests Cell lines and animal models Preclinical drug development Preclinical development of new drug encompasses(包 含) all of the activities that must take place before a new chemical entity can be administered to human. 2019/8/28 Institute of clinical pharmacology, AHMU 8 Optimal drug candidate Clinical testing development Human

Generally,preclinical drug development includes three areas (1)Vitro studies,药理学特性 (2)Drug supply and formulation (3)Vivo studies in animal models. 治疗原则证据和蘑在临床疗减 2019/8/28 Institute of clinical pharmacology,AHMU 9
Generally, preclinical drug development includes three areas : (1) Vitro studies, 药理学特性 (2) Drug supply and formulation , (3) Vivo studies in animal models. 2019/8/28 Institute of clinical pharmacology, AHMU 9 治疗原则证据和潜在临床疗效

1.In Vitro Studies (1)Vitro screening assays are to study the ability of drugs: To bind to receptors, To inhibit specific enzymes, To stabilize or destabilize multi protein complexes. URP "Looks like Carstairs finally got a single cell to eat a whole meal By the way,have you seen Carstairs lately?" 2019/8/28 Institute of clinical pharmacology,AHMU 10
1. In Vitro Studies (1) Vitro screening assays are to study the ability of drugs: To bind to receptors, To inhibit specific enzymes, To stabilize or destabilize multi protein complexes. 2019/8/28 Institute of clinical pharmacology, AHMU 10