
English Reading Materials Chapter 19:Agents Used in Cardiac Arrhythmias INTRODUCTION Cardiac arrhythmias are a frequent problem in clinical practice,occurring in up to 25%of patients treated with digitalis,50%of anesthetized patients,and over 80%of patients with acute myocardial infarction.Arrhythmias may require treatment because rhythms that are too rapid,too slow,or asynchronous can reduce cardiac output.Some arrhythmias can precipitate more serious or even lethal rhythm disturbances eg, early premature ventricular depolarizations can precipitate ventricular fibrillation.In such patients,antiarrhythmic drugs may be lifesaving.On the other hand,the hazards of antiarrhythmic drugs and in particular the fact that they can precipitate lethal arrhythmias in some patients has led to a reevaluation of their relative risks and benefits.In general,treatment of asymptomatic or minimally symptomatic arrhythmias should be avoided for this reason. Arrhythmias can be treated with the drugs discussed in this chapter and with nonpharmacologic therapies such as pacemakers,cardioversion,catheter ablation,and surgery.This chapter describes the pharmacology of drugs that suppress arrhythmias by a direct action on the cardiac cell membrane.Other modes of therapy are discussed briefly(see Box,The Nonpharmacologic Therapy of Cardiac Arrhythmias) THE NONPHARMACOLOGIC THERAPY OF CARDIAC ARRHYTHMIAS It was recognized over 100 years ago that reentry in simple in vitro models (eg,rings of conducting tissues)was permanently interrupted by transecting the reentry circuit. This concept is now applied in cardiac arrhythmias with defined anatomic pathways eg,atrioventricular reentry using accessory pathways,atrioventricular node reentry,atrial flutter,and some forms of ventricular tachycardia by treatment with radiofrequency catheter ablation.Recent studies have shown that paroxysmal and persistent atrial fibrillation may arise from one of the pulmonary veins.Both forms of atrial fibrillation can be cured by electrically isolating the pulmonary veins by radiofrequency catheter ablation or during concomitant cardiac surgery. Another form of nonpharmacologic therapy is the implantable cardioverter-defibrillator (ICD),a device that can automatically detect and treat potentially fatal arrhythmias such as ventricular fibrillation.ICDs are now widely used in patients who have been resuscitated from such arrhythmias,and several trials have shown that ICD treatment reduces mortality in patients with coronary artery 1
1 English Reading Materials Chapter 19: Agents Used in Cardiac Arrhythmias INTRODUCTION Cardiac arrhythmias are a frequent problem in clinical practice, occurring in up to 25% of patients treated with digitalis, 50% of anesthetized patients, and over 80% of patients with acute myocardial infarction. Arrhythmias may require treatment because rhythms that are too rapid, too slow, or asynchronous can reduce cardiac output. Some arrhythmias can precipitate more serious or even lethal rhythm disturbances eg, early premature ventricular depolarizations can precipitate ventricular fibrillation. In such patients, antiarrhythmic drugs may be lifesaving. On the other hand, the hazards of antiarrhythmic drugs and in particular the fact that they can precipitate lethal arrhythmias in some patients has led to a reevaluation of their relative risks and benefits. In general, treatment of asymptomatic or minimally symptomatic arrhythmias should be avoided for this reason. Arrhythmias can be treated with the drugs discussed in this chapter and with nonpharmacologic therapies such as pacemakers, cardioversion, catheter ablation, and surgery. This chapter describes the pharmacology of drugs that suppress arrhythmias by a direct action on the cardiac cell membrane. Other modes of therapy are discussed briefly (see Box, The Nonpharmacologic Therapy of Cardiac Arrhythmias). THE NONPHARMACOLOGIC THERAPY OF CARDIAC ARRHYTHMIAS It was recognized over 100 years ago that reentry in simple in vitro models (eg, rings of conducting tissues) was permanently interrupted by transecting the reentry circuit. This concept is now applied in cardiac arrhythmias with defined anatomic pathways eg, atrioventricular reentry using accessory pathways, atrioventricular node reentry, atrial flutter, and some forms of ventricular tachycardia by treatment with radiofrequency catheter ablation. Recent studies have shown that paroxysmal and persistent atrial fibrillation may arise from one of the pulmonary veins. Both forms of atrial fibrillation can be cured by electrically isolating the pulmonary veins by radiofrequency catheter ablation or during concomitant cardiac surgery. Another form of nonpharmacologic therapy is the implantable cardioverter-defibrillator (ICD), a device that can automatically detect and treat potentially fatal arrhythmias such as ventricular fibrillation. ICDs are now widely used in patients who have been resuscitated from such arrhythmias, and several trials have shown that ICD treatment reduces mortality in patients with coronary artery

disease who have an ejection fraction 30%and in patients with class 2 or 3 heart failure and no prior history of arrhythmias.The increasing use of nonpharmacologic antiarrhythmic therapies reflects both advances in the relevant technologies and an increasing appreciation of the dangers of long-term therapy with currently available drugs. ELECTROPHYSIOLOGY OF NORMAL CARDIAC RHYTHM Introduction The electrical impulse that triggers a normal cardiac contraction originates at regular intervals in the sinoatrial node(Figure 1),usually at a frequency of 60-100 beats per minute.This impulse spreads rapidly through the atria and enters the atrioventricular node,which is normally the only conduction pathway between the atria and ventricles. Conduction through the atrioventricular node is slow,requiring about 0.15 s.(This delay provides time for atrial contraction to propel blood into the ventricles.)The impulse then propagates over the His-Purkinje system and invades all parts of the ventricles,beginning with the endocardial surface near the apex and ending with the epicardial surface at the base of the heart.Ventricular activation is complete in less than 0.1 s;therefore,contraction of all of the ventricular muscle is normally synchronous and hemodynamically effective. Arrhythmias consist of cardiac depolarizations that deviate from the above description in one or more aspects ie,there is an abnormality in the site oforigin of the impulse,its rate or regularity,or its conduction. 2
2 disease who have an ejection fraction 30% and in patients with class 2 or 3 heart failure and no prior history of arrhythmias. The increasing use of nonpharmacologic antiarrhythmic therapies reflects both advances in the relevant technologies and an increasing appreciation of the dangers of long-term therapy with currently available drugs. ELECTROPHYSIOLOGY OF NORMAL CARDIAC RHYTHM Introduction The electrical impulse that triggers a normal cardiac contraction originates at regular intervals in the sinoatrial node (Figure 1), usually at a frequency of 60-100 beats per minute. This impulse spreads rapidly through the atria and enters the atrioventricular node, which is normally the only conduction pathway between the atria and ventricles. Conduction through the atrioventricular node is slow, requiring about 0.15 s. (This delay provides time for atrial contraction to propel blood into the ventricles.) The impulse then propagates over the His-Purkinje system and invades all parts of the ventricles, beginning with the endocardial surface near the apex and ending with the epicardial surface at the base of the heart. Ventricular activation is complete in less than 0.1 s; therefore, contraction of all of the ventricular muscle is normally synchronous and hemodynamically effective. Arrhythmias consist of cardiac depolarizations that deviate from the above description in one or more aspects ie, there is an abnormality in the site of origin of the impulse, its rate or regularity, or its conduction

Superior vena cava Phase 0 SA node Atrium AV node Overshoot 0 Phase 0 mV Purkinje -100 Tricuspid Resting potentia valve Mitral valve Action potential phases Ventricle 0:Upstroke 1:Early-fast repolarization 2:Plateau 3:Repolarization 4:Diastole ECG Q S PR- QT 200ms Figure 1.Schematic representation of the heart and normal cardiac electrical activity (intracellular recordings from areas indicated and ECG).Sinoatrial node, atrioventricular node,and Purkinje cells display pacemaker activity (phase 4 depolarization).The ECG is the body surface manifestation of the depolarization and repolarization waves of the heart.The P wave is generated by atrial depolarization, the QRS by ventricular muscle depolarization,and the T wave by ventricular repolarization.Thus,the PR interval is a measure of conduction time from atrium to ventricle,and the QRS duration indicates the time required for all of the ventricular cells to be activated (ie,the intraventricular conduction time).The QT interval reflects the duration of the ventricular action potential. Ionic Basis of Membrane Electrical Activity The transmembrane potential of cardiac cells is determined by the concentrations of several ions chiefly sodium (Na),potassium (K),calcium (Ca2),and chloride (Cl)on either side of the membrane and the permeability of the membrane to each ion.These water-soluble ions are unable to freely diffuse across the lipid cell membrane in response to their electrical and concentration gradients;they require aqueous channels(specific pore-forming proteins)for such diffusion.Thus,ions move across cell membranes in response to their gradients only at specific times during the 3
3 Figure 1. Schematic representation of the heart and normal cardiac electrical activity (intracellular recordings from areas indicated and ECG). Sinoatrial node, atrioventricular node, and Purkinje cells display pacemaker activity (phase 4 depolarization). The ECG is the body surface manifestation of the depolarization and repolarization waves of the heart. The P wave is generated by atrial depolarization, the QRS by ventricular muscle depolarization, and the T wave by ventricular repolarization. Thus, the PR interval is a measure of conduction time from atrium to ventricle, and the QRS duration indicates the time required for all of the ventricular cells to be activated (ie, the intraventricular conduction time). The QT interval reflects the duration of the ventricular action potential. Ionic Basis of Membrane Electrical Activity The transmembrane potential of cardiac cells is determined by the concentrations of several ions chiefly sodium (Na+ ), potassium (K+ ), calcium (Ca2+), and chloride (Cl- ) on either side of the membrane and the permeability of the membrane to each ion. These water-soluble ions are unable to freely diffuse across the lipid cell membrane in response to their electrical and concentration gradients; they require aqueous channels (specific pore-forming proteins) for such diffusion. Thus, ions move across cell membranes in response to their gradients only at specific times during the

cardiac cycle when these ion channels are open.The movements of the ions produce currents that form the basis of the cardiac action potential.Individual channels are relatively ion-specific,and the flux of ions through them is thought to be controlled by "gates"(probably flexible peptide chains or energy barriers).Each type of channel has its own type of gate(sodium,calcium,and some potassium channels are each thought to have two types of gates),and each type of gate is opened and closed by specific transmembrane voltage,ionic,or metabolic conditions. At rest,most cells are not significantly permeable to sodium,but at the start of each action potential,they become quite permeable (see below).In electrophysiologic terms,the conductance of the fast sodium channel suddenly increases in response to a depolarizing stimulus.Similarly,calcium enters and potassium leaves the cell with each action potential.Therefore,in addition to ion channels,the cell must have mechanisms to maintain stable transmembrane ionic conditions by establishing and maintaining ion gradients.The most important of these active mechanisms is the sodium pump,Na/K ATPase.This pump and other active ion carriers contribute indirectly to the transmembrane potential by maintaining the gradients necessary for diffusion through channels.In addition,some pumps and exchangers produce net current flow(eg,by exchanging three Na"for two K*ions)and hence are termed "electrogenic." When the cardiac cell membrane becomes permeable to a specific ion (ie when the channels selective for that ion are open),movement of that ion across the cell membrane is determined by Ohm's law:current voltage resistance,or current voltage conductance.Conductance is determined by the properties of the individual ion channel protein.The voltage term is the difference between the actual membrane potential and the reversal potential for that ion(the membrane potential at which no current would flow even if channels were open).For example,in the case of sodium in a cardiac cell at rest,there is a substantial concentration gradient (140 mmol/L Na"outside;10-15 mmol/L Na"inside)and an electrical gradient (0 mV outside:-90 mV inside)that would drive Na'into cells.Sodium does not enter the cell at rest because sodium channels are closed;when sodium channels open,the very large influx of Na'ions accounts for phase 0 depolarization.The situation for K*ions in the resting cardiac cell is quite different.Here,the concentration gradient (140 mmol/L inside;4 mmol/L outside)would drive the ion out of the cell,but the electrical gradient would drive it in;that is,the inward gradient is in equilibrium with the outward gradient.In fact,certain potassium channels("inward rectifier"channels) are open in the resting cell,but little current flows through them because of this balance.The equilibrium,or reversal potential,for ions is determined by the Nernst equation: where Ce and Ci are the extracellular and intracellular concentrations,respectively, multiplied by their activity coefficients.Note that raising extracellular potassium makes Ek less negative.When this occurs,the membrane depolarizes until the new Ek 4
4 cardiac cycle when these ion channels are open. The movements of the ions produce currents that form the basis of the cardiac action potential. Individual channels are relatively ion-specific, and the flux of ions through them is thought to be controlled by "gates" (probably flexible peptide chains or energy barriers). Each type of channel has its own type of gate (sodium, calcium, and some potassium channels are each thought to have two types of gates), and each type of gate is opened and closed by specific transmembrane voltage, ionic, or metabolic conditions. At rest, most cells are not significantly permeable to sodium, but at the start of each action potential, they become quite permeable (see below). In electrophysiologic terms, the conductance of the fast sodium channel suddenly increases in response to a depolarizing stimulus. Similarly, calcium enters and potassium leaves the cell with each action potential. Therefore, in addition to ion channels, the cell must have mechanisms to maintain stable transmembrane ionic conditions by establishing and maintaining ion gradients. The most important of these active mechanisms is the sodium pump, Na+ /K+ ATPase. This pump and other active ion carriers contribute indirectly to the transmembrane potential by maintaining the gradients necessary for diffusion through channels. In addition, some pumps and exchangers produce net current flow (eg, by exchanging three Na+ for two K+ ions) and hence are termed "electrogenic." When the cardiac cell membrane becomes permeable to a specific ion (ie when the channels selective for that ion are open), movement of that ion across the cell membrane is determined by Ohm's law: current = voltage resistance, or current = voltage conductance. Conductance is determined by the properties of the individual ion channel protein. The voltage term is the difference between the actual membrane potential and the reversal potential for that ion (the membrane potential at which no current would flow even if channels were open). For example, in the case of sodium in a cardiac cell at rest, there is a substantial concentration gradient (140 mmol/L Na+ outside; 10-15 mmol/L Na+ inside) and an electrical gradient (0 mV outside; -90 mV inside) that would drive Na+ into cells. Sodium does not enter the cell at rest because sodium channels are closed; when sodium channels open, the very large influx of Na+ ions accounts for phase 0 depolarization. The situation for K+ ions in the resting cardiac cell is quite different. Here, the concentration gradient (140 mmol/L inside; 4 mmol/L outside) would drive the ion out of the cell, but the electrical gradient would drive it in; that is, the inward gradient is in equilibrium with the outward gradient. In fact, certain potassium channels ("inward rectifier" channels) are open in the resting cell, but little current flows through them because of this balance. The equilibrium, or reversal potential, for ions is determined by the Nernst equation: where Ce and Ci are the extracellular and intracellular concentrations, respectively, multiplied by their activity coefficients. Note that raising extracellular potassium makes EK less negative. When this occurs, the membrane depolarizes until the new EK

is reached.Thus,extracellular potassium concentration and inward rectifier channel function are the major factors determining the membrane potential of the resting cardiac cell.The conditions required for application of the Nernst equation are approximated at the peak of the overshoot (using sodium concentrations)and during rest (using potassium concentrations)in most nonpacemaker cardiac cells.If the permeability is significant for both potassium and sodium,the Nernst equation is not a good predictor of membrane potential,but the Goldman-Hodgkin-Katz equation may be used: In pacemaker cells (whether normal or ectopic),spontaneous depolarization (the pacemaker potential)occurs during diastole (phase 4,Figure 1).This depolarization results from a gradual increase of depolarizing current through special hyperpolarization-activated ion channels in pacemaker cells.The effect of changing extracellular potassium is more complex in a pacemaker cell than it is in a nonpacemaker cell because the effect on permeability to potassium is much more important in a pacemaker.In a pacemaker especially an ectopic one the end result of an increase in extracellular potassium will usually be to slow or stop the pacemaker. Conversely,hypokalemia will often facilitate ectopic pacemakers. EFFECTS OF POTASSIUM The effects of changes in serum potassium on cardiac action potential duration, pacemaker rate,and arrhythmias can appear somewhat paradoxical if changes are predicted based solely on a consideration of changes in the potassium electrochemical gradient.In the heart,however,changes in serum potassium concentration have the additional effect of altering potassium conductance (increased extracellular potassium increases potassium conductance)independent of simple changes in electrochemical driving force,and this effect often predominates.As a result,the actual observed effects of hyperkalemia include reduced action potential duration,slowed conduction, decreased pacemaker rate,and decreased pacemaker arrhythmogenesis.Conversely, the actual observed effects of hypokalemia include prolonged action potential duration, increased pacemaker rate,and increased pacemaker arrhythmogenesis.Furthermore, pacemaker rate and arrhythmias involving ectopic pacemaker cells appear to be more sensitive to changes in serum potassium concentration,compared with cells of the sinoatrial node.These effects of serum potassium on the heart probably contribute to the observed increased sensitivity to potassium channel-blocking antiarrhythmic agents (quinidine or sotalol)during hypokalemia,eg,accentuated action potential prolongation and tendency to cause torsade de pointes. The Active Cell Membrane 5
5 is reached. Thus, extracellular potassium concentration and inward rectifier channel function are the major factors determining the membrane potential of the resting cardiac cell. The conditions required for application of the Nernst equation are approximated at the peak of the overshoot (using sodium concentrations) and during rest (using potassium concentrations) in most nonpacemaker cardiac cells. If the permeability is significant for both potassium and sodium, the Nernst equation is not a good predictor of membrane potential, but the Goldman-Hodgkin-Katz equation may be used: In pacemaker cells (whether normal or ectopic), spontaneous depolarization (the pacemaker potential) occurs during diastole (phase 4, Figure 1). This depolarization results from a gradual increase of depolarizing current through special hyperpolarization-activated ion channels in pacemaker cells. The effect of changing extracellular potassium is more complex in a pacemaker cell than it is in a nonpacemaker cell because the effect on permeability to potassium is much more important in a pacemaker. In a pacemaker especially an ectopic one the end result of an increase in extracellular potassium will usually be to slow or stop the pacemaker. Conversely, hypokalemia will often facilitate ectopic pacemakers. EFFECTS OF POTASSIUM The effects of changes in serum potassium on cardiac action potential duration, pacemaker rate, and arrhythmias can appear somewhat paradoxical if changes are predicted based solely on a consideration of changes in the potassium electrochemical gradient. In the heart, however, changes in serum potassium concentration have the additional effect of altering potassium conductance (increased extracellular potassium increases potassium conductance) independent of simple changes in electrochemical driving force, and this effect often predominates. As a result, the actual observed effects of hyperkalemia include reduced action potential duration, slowed conduction, decreased pacemaker rate, and decreased pacemaker arrhythmogenesis. Conversely, the actual observed effects of hypokalemia include prolonged action potential duration, increased pacemaker rate, and increased pacemaker arrhythmogenesis. Furthermore, pacemaker rate and arrhythmias involving ectopic pacemaker cells appear to be more sensitive to changes in serum potassium concentration, compared with cells of the sinoatrial node. These effects of serum potassium on the heart probably contribute to the observed increased sensitivity to potassium channel-blocking antiarrhythmic agents (quinidine or sotalol) during hypokalemia, eg, accentuated action potential prolongation and tendency to cause torsade de pointes. The Active Cell Membrane

In normal atrial,Purkinje,and ventricular cells,the action potential upstroke (phase 0) is dependent on sodium current.From a functional point of view,it is convenient to describe the behavior of the sodium current in terms of three channel states(Figure 2). The cardiac sodium channel protein has been cloned,and it is now recognized that these channel states actually represent different protein conformations.In addition, regions of the protein that confer specific behaviors,such as voltage sensing,pore formation,and inactivation,are now being identified.The gates described below and in Figure 2 represent such regions. Depolarization to the threshold voltage results in opening of the activation (m)gates of sodium channels(Figure 2,middle).If the inactivation (h)gates of these channels have not already closed,the channels are now open or activated,and sodium permeability is markedly increased,greatly exceeding the permeability for any other ion.Extracellular sodium therefore diffuses down its electrochemical gradient into the cell,and the membrane potential very rapidly approaches the sodium equilibrium potential,ENa (about +70 mV when Nae 140 mmol/L and Nai 10 mmol/L).This intense sodium current is very brief because opening of the m gates upon depolarization is promptly followed by closure of the h gates and inactivation of the sodium channels(Figure 2,right). Most calcium channels become activated and inactivated in what appears to be the same way as sodium channels,but in the case of the most common type of cardiac calcium channel (the "L"type),the transitions occur more slowly and at more positive potentials.The action potential plateau(phases 1 and 2)reflects the turning off of most of the sodium current,the waxing and waning of calcium current,and the slow development of a repolarizing potassium current. Final repolarization (phase 3)of the action potential results from completion of sodium and calcium channel inactivation and the growth of potassium permeability, so that the membrane potential once again approaches the potassium equilibrium potential.The major potassium currents involved in phase 3 repolarization include a rapidly activating potassium current(Ik)and a slowly activating potassium current (Iks).These two potassium currents are sometimes discussed together as "Ik."These processes are diagrammed in Figure 3.It is noteworthy that a different potassium current,distinct from Ikr and Iks,may control repolarization in sinoatrial nodal cells. This explains why some drugs that block either Ikr or Iks may prolong repolarization in Purkinje and ventricular cells,but have little effect on sinoatrial nodal repolarization. 6
6 In normal atrial, Purkinje, and ventricular cells, the action potential upstroke (phase 0) is dependent on sodium current. From a functional point of view, it is convenient to describe the behavior of the sodium current in terms of three channel states (Figure 2). The cardiac sodium channel protein has been cloned, and it is now recognized that these channel states actually represent different protein conformations. In addition, regions of the protein that confer specific behaviors, such as voltage sensing, pore formation, and inactivation, are now being identified. The gates described below and in Figure 2 represent such regions. Depolarization to the threshold voltage results in opening of the activation (m) gates of sodium channels (Figure 2, middle). If the inactivation (h) gates of these channels have not already closed, the channels are now open or activated, and sodium permeability is markedly increased, greatly exceeding the permeability for any other ion. Extracellular sodium therefore diffuses down its electrochemical gradient into the cell, and the membrane potential very rapidly approaches the sodium equilibrium potential, ENa (about +70 mV when Nae = 140 mmol/L and Nai = 10 mmol/L). This intense sodium current is very brief because opening of the m gates upon depolarization is promptly followed by closure of the h gates and inactivation of the sodium channels (Figure 2, right). Most calcium channels become activated and inactivated in what appears to be the same way as sodium channels, but in the case of the most common type of cardiac calcium channel (the "L" type), the transitions occur more slowly and at more positive potentials. The action potential plateau (phases 1 and 2) reflects the turning off of most of the sodium current, the waxing and waning of calcium current, and the slow development of a repolarizing potassium current. Final repolarization (phase 3) of the action potential results from completion of sodium and calcium channel inactivation and the growth of potassium permeability, so that the membrane potential once again approaches the potassium equilibrium potential. The major potassium currents involved in phase 3 repolarization include a rapidly activating potassium current (IKr) and a slowly activating potassium current (IKs). These two potassium currents are sometimes discussed together as "IK." These processes are diagrammed in Figure 3. It is noteworthy that a different potassium current, distinct from IKr and IKs, may control repolarization in sinoatrial nodal cells. This explains why some drugs that block either IKr or IKs may prolong repolarization in Purkinje and ventricular cells, but have little effect on sinoatrial nodal repolarization
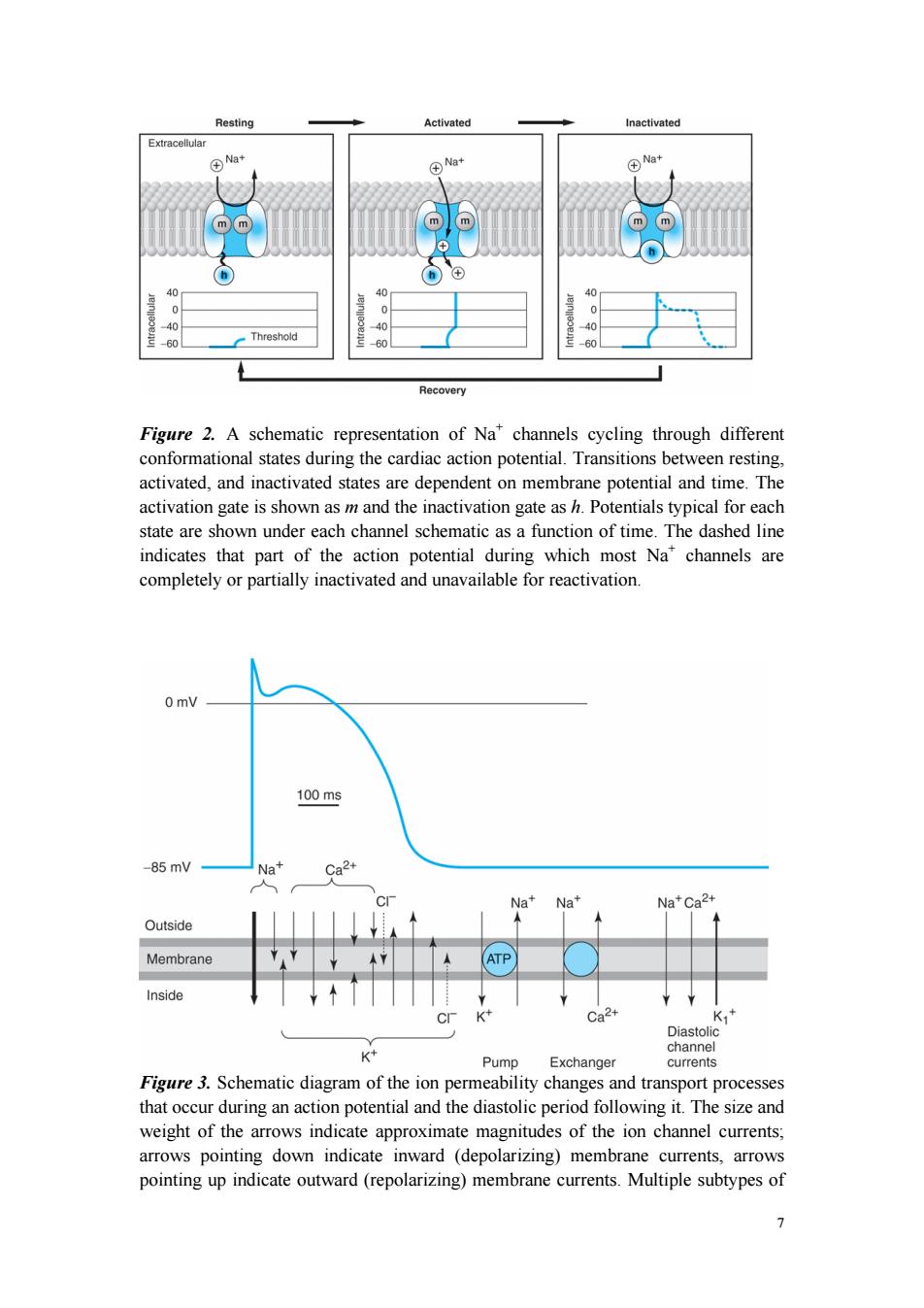
Resting Activated Inactivated Extracellular Na+ ④N+ m m ⑧ 40 0 0 0 90 0 60 Threshold 60 60 Recovery Figure 2.A schematic representation of Na*channels cycling through different conformational states during the cardiac action potential.Transitions between resting, activated,and inactivated states are dependent on membrane potential and time.The activation gate is shown as m and the inactivation gate as h.Potentials typical for each state are shown under each channel schematic as a function of time.The dashed line indicates that part of the action potential during which most Na"channels are completely or partially inactivated and unavailable for reactivation. 0 mV 100ms -85mV Na+ Ca2+ Na+Na+ Na+Ca2+ Outside Membrane ATP Inside Ca2+ K,+ Diastoli K channel Pump Exchanger currents Figure 3.Schematic diagram of the ion permeability changes and transport processes that occur during an action potential and the diastolic period following it.The size and weight of the arrows indicate approximate magnitudes of the ion channel currents; arrows pointing down indicate inward (depolarizing)membrane currents,arrows pointing up indicate outward(repolarizing)membrane currents.Multiple subtypes of 1
7 Figure 2. A schematic representation of Na+ channels cycling through different conformational states during the cardiac action potential. Transitions between resting, activated, and inactivated states are dependent on membrane potential and time. The activation gate is shown as m and the inactivation gate as h. Potentials typical for each state are shown under each channel schematic as a function of time. The dashed line indicates that part of the action potential during which most Na+ channels are completely or partially inactivated and unavailable for reactivation. Figure 3. Schematic diagram of the ion permeability changes and transport processes that occur during an action potential and the diastolic period following it. The size and weight of the arrows indicate approximate magnitudes of the ion channel currents; arrows pointing down indicate inward (depolarizing) membrane currents, arrows pointing up indicate outward (repolarizing) membrane currents. Multiple subtypes of

potassium and calcium currents,with different sensitivities to blocking drugs,have been identified.Chloride currents (dotted arrows)produce both inward and outward membrane currents during the cardiac action potential. The Effect of Resting Potential on Action Potentials A key factor in the pathophysiology of arrhythmias and the actions of antiarrhythmic drugs is the relationship between the resting potential of a cell and the action potentials that can be evoked in it(Figure 4,left panel).Because the inactivation gates of sodium channels in the resting membrane close over the potential range-75 to-55 mV,fewer sodium channels are "available"for diffusion of sodium ions when an action potential is evoked from a resting potential of-60 mV than when it is evoked from a resting potential of-80 mV.Important consequences of the reduction in peak sodium permeability include reduced maximum upstroke velocity (for maximum rate of change of membrane voltage),reduced action potential amplitude,reduced excitability,and reduced conduction velocity. During the plateau of the action potential,most sodium channels are inactivated. Upon repolarization,recovery from inactivation takes place (in the terminology of Figure 2,the h gates reopen),making the channels again available for excitation.The time between phase 0 and sufficient recovery of sodium channels in phase 3 to permit a new propagated response to an external stimulus is the refractory period.Changes in refractoriness (determined by either altered recovery from inactivation or altered action potential duration)can be important in the genesis or suppression of certain arrhythmias.Another important effect of less negative resting potential is prolongation of this recovery time,as shown in Figure 4 (right panel).The prolongation of recovery time is reflected in an increase in the effective refractory period. A brief,sudden,depolarizing stimulus,whether caused by a propagating action potential or by an external electrode arrangement,causes the opening of large numbers of activation gates before a significant number of inactivation gates can close. In contrast,slow reduction (depolarization)of the resting potential,whether brought about by hyperkalemia,sodium pump blockade,or ischemic cell damage,results in depressed sodium currents during the upstrokes of action potentials.Depolarization of the resting potential to levels positive to -55 mV abolishes sodium currents,since all sodium channels are inactivated.However,such severely depolarized cells have been found to support special action potentials under circumstances that increase calcium permeability or decrease potassium permeability.These "slow responses"-slow upstroke velocity and slow conductiondepend on a calcium inward current and constitute the normal electrical activity in the sinoatrial and atrioventricular nodes, since these tissues have a normal resting potential in the range of-50 to-70 mV.Slow responses may also be important for certain arrhythmias.Modern techniques of molecular biology and electrophysiology can identify multiple subtypes of calcium 8
8 potassium and calcium currents, with different sensitivities to blocking drugs, have been identified. Chloride currents (dotted arrows) produce both inward and outward membrane currents during the cardiac action potential. The Effect of Resting Potential on Action Potentials A key factor in the pathophysiology of arrhythmias and the actions of antiarrhythmic drugs is the relationship between the resting potential of a cell and the action potentials that can be evoked in it (Figure 4, left panel). Because the inactivation gates of sodium channels in the resting membrane close over the potential range -75 to -55 mV, fewer sodium channels are "available" for diffusion of sodium ions when an action potential is evoked from a resting potential of -60 mV than when it is evoked from a resting potential of -80 mV. Important consequences of the reduction in peak sodium permeability include reduced maximum upstroke velocity (for maximum rate of change of membrane voltage), reduced action potential amplitude, reduced excitability, and reduced conduction velocity. During the plateau of the action potential, most sodium channels are inactivated. Upon repolarization, recovery from inactivation takes place (in the terminology of Figure 2, the h gates reopen), making the channels again available for excitation. The time between phase 0 and sufficient recovery of sodium channels in phase 3 to permit a new propagated response to an external stimulus is the refractory period. Changes in refractoriness (determined by either altered recovery from inactivation or altered action potential duration) can be important in the genesis or suppression of certain arrhythmias. Another important effect of less negative resting potential is prolongation of this recovery time, as shown in Figure 4 (right panel). The prolongation of recovery time is reflected in an increase in the effective refractory period. A brief, sudden, depolarizing stimulus, whether caused by a propagating action potential or by an external electrode arrangement, causes the opening of large numbers of activation gates before a significant number of inactivation gates can close. In contrast, slow reduction (depolarization) of the resting potential, whether brought about by hyperkalemia, sodium pump blockade, or ischemic cell damage, results in depressed sodium currents during the upstrokes of action potentials. Depolarization of the resting potential to levels positive to -55 mV abolishes sodium currents, since all sodium channels are inactivated. However, such severely depolarized cells have been found to support special action potentials under circumstances that increase calcium permeability or decrease potassium permeability. These "slow responses" - slow upstroke velocity and slow conductiondepend on a calcium inward current and constitute the normal electrical activity in the sinoatrial and atrioventricular nodes, since these tissues have a normal resting potential in the range of -50 to -70 mV. Slow responses may also be important for certain arrhythmias. Modern techniques of molecular biology and electrophysiology can identify multiple subtypes of calcium

and potassium channels.One way in which such subtypes may differ is in sensitivity to drug effects,so drugs targeting specific channel subtypes may be developed in the future. 里10,00 Drug 100 10.000 Control 1000 Drug 100 0 Contro -120-100-80 60 -120-100-80 60 Resting membrane potential(mV) Resting membrane potential(mV) Figure 4.Dependence of sodium channel function on the membrane potential preceding the stimulus.Left:The fraction of sodium channels available for opening in response to a stimulus is determined by the membrane potential immediately preceding the stimulus.The decrease in the fraction available when the resting potential is depolarized in the absence of a drug (control curve)results from the voltage-dependent closure of h gates in the channels.The curve labeled Drug illustrates the effect of a typical local anesthetic antiarrhythmic drug.Most sodium channels are inactivated during the plateau of the action potential.Right:The time constant for recovery from inactivation after repolarization also depends on the resting potential.In the absence of drug,recovery occurs in less than 10 ms at normal resting potentials (-85 to -95 mV).Depolarized cells recover more slowly (note logarithmic scale).In the presence of a sodium channel-blocking drug,the time constant of recovery is increased,but the increase is far greater at depolarized potentials than at more negative ones. MECHANISMS OF ARRHYTHMIAS Introduction Many factors can precipitate or exacerbate arrhythmias:ischemia,hypoxia,acidosis or alkalosis,electrolyte abnormalities,excessive catecholamine exposure,autonomic influences,drug toxicity (eg,digitalis or antiarrhythmic drugs),overstretching of cardiac fibers,and the presence of scarred or otherwise diseased tissue.However,all arrhythmias result from (1)disturbances in impulse formation,(2)disturbances in impulse conduction,or(3)both. 9
9 and potassium channels. One way in which such subtypes may differ is in sensitivity to drug effects, so drugs targeting specific channel subtypes may be developed in the future. Figure 4. Dependence of sodium channel function on the membrane potential preceding the stimulus. Left: The fraction of sodium channels available for opening in response to a stimulus is determined by the membrane potential immediately preceding the stimulus. The decrease in the fraction available when the resting potential is depolarized in the absence of a drug (control curve) results from the voltage-dependent closure of h gates in the channels. The curve labeled Drug illustrates the effect of a typical local anesthetic antiarrhythmic drug. Most sodium channels are inactivated during the plateau of the action potential. Right: The time constant for recovery from inactivation after repolarization also depends on the resting potential. In the absence of drug, recovery occurs in less than 10 ms at normal resting potentials (-85 to -95 mV). Depolarized cells recover more slowly (note logarithmic scale). In the presence of a sodium channel-blocking drug, the time constant of recovery is increased, but the increase is far greater at depolarized potentials than at more negative ones. MECHANISMS OF ARRHYTHMIAS Introduction Many factors can precipitate or exacerbate arrhythmias: ischemia, hypoxia, acidosis or alkalosis, electrolyte abnormalities, excessive catecholamine exposure, autonomic influences, drug toxicity (eg, digitalis or antiarrhythmic drugs), overstretching of cardiac fibers, and the presence of scarred or otherwise diseased tissue. However, all arrhythmias result from (1) disturbances in impulse formation, (2) disturbances in impulse conduction, or (3) both

Disturbances of Impulse Formation The interval between depolarizations of a pacemaker cell is the sum of the duration of the action potential and the duration of the diastolic interval.Shortening of either duration results in an increase in pacemaker rate.The more important of the two, diastolic interval,is determined primarily by the slope of phase 4 depolarization (pacemaker potential).Vagal discharge and -receptor-blocking drugs slow normal pacemaker rate by reducing the phase 4 slope(acetylcholine also makes the maximum diastolic potential more negative).Acceleration of pacemaker discharge is often brought about by increased phase 4 depolarization slope,which can be caused by hypokalemia,-adrenoceptor stimulation,positive chronotropic drugs,fiber stretch, acidosis,and partial depolarization by currents of injury. Latent pacemakers(cells that show slow phase 4 depolarization even under normal conditions,eg,some Purkinje fibers)are particularly prone to acceleration by the above mechanisms.However,all cardiac cells,including normally quiescent atrial and ventricular cells,may show repetitive pacemaker activity when depolarized under appropriate conditions,especially if hypokalemia is also present. Afterdepolarizations (Figure 5)are depolarizations that interrupt phase 3 (early afterdepolarizations,EADs)or phase 4 (delayed afterdepolarizations,DADs). EADs are usually exacerbated at slow heart rates and are thought to contribute to the development of long QT-related arrhythmias.DADs on the other hand,often occur when intracellular calcium is increased.They are exacerbated by fast heart rates and are thought to be responsible for some arrhythmias related to digitalis excess,to catecholamines,and to myocardial ischemia. MOLECULAR GENETIC BASIS OF CARDIAC ARRHYTHMIAS It is now possible to define the molecular basis of several congenital and acquired cardiac arrhythmias.The best example is the polymorphic ventricular tachycardia known as torsade de pointes (shown in Figure 7),which is associated with prolongation of the QT interval (especially at the onset of the tachycardia),syncope, and sudden death.This must represent prolongation of the action potential of at least some ventricular cells (Figure 1).The effect can,in theory,be attributed either to increased inward current (gain of function)or decreased outward current (loss of function)during the plateau of the action potential.In fact,recent molecular genetic studies have identified up to 300 different mutations in at least eight ion channel genes that produce the congenital long QT (LQT)syndrome (Table 1),and each mutation may have different clinical implications.Loss of function mutations in potassium channel genes produce decreases in outward repolarizing current and are responsible for LQT subtypes 1,2,5,6,and 7.HERG and KCNE2 (MiRPI)genes 10
10 Disturbances of Impulse Formation The interval between depolarizations of a pacemaker cell is the sum of the duration of the action potential and the duration of the diastolic interval. Shortening of either duration results in an increase in pacemaker rate. The more important of the two, diastolic interval, is determined primarily by the slope of phase 4 depolarization (pacemaker potential). Vagal discharge and -receptor-blocking drugs slow normal pacemaker rate by reducing the phase 4 slope (acetylcholine also makes the maximum diastolic potential more negative). Acceleration of pacemaker discharge is often brought about by increased phase 4 depolarization slope, which can be caused by hypokalemia, -adrenoceptor stimulation, positive chronotropic drugs, fiber stretch, acidosis, and partial depolarization by currents of injury. Latent pacemakers (cells that show slow phase 4 depolarization even under normal conditions, eg, some Purkinje fibers) are particularly prone to acceleration by the above mechanisms. However, all cardiac cells, including normally quiescent atrial and ventricular cells, may show repetitive pacemaker activity when depolarized under appropriate conditions, especially if hypokalemia is also present. Afterdepolarizations (Figure 5) are depolarizations that interrupt phase 3 (early afterdepolarizations, EADs) or phase 4 (delayed afterdepolarizations, DADs). EADs are usually exacerbated at slow heart rates and are thought to contribute to the development of long QT-related arrhythmias. DADs on the other hand, often occur when intracellular calcium is increased. They are exacerbated by fast heart rates and are thought to be responsible for some arrhythmias related to digitalis excess, to catecholamines, and to myocardial ischemia. MOLECULAR GENETIC BASIS OF CARDIAC ARRHYTHMIAS It is now possible to define the molecular basis of several congenital and acquired cardiac arrhythmias. The best example is the polymorphic ventricular tachycardia known as torsade de pointes (shown in Figure 7), which is associated with prolongation of the QT interval (especially at the onset of the tachycardia), syncope, and sudden death. This must represent prolongation of the action potential of at least some ventricular cells (Figure 1). The effect can, in theory, be attributed either to increased inward current (gain of function) or decreased outward current (loss of function) during the plateau of the action potential. In fact, recent molecular genetic studies have identified up to 300 different mutations in at least eight ion channel genes that produce the congenital long QT (LQT) syndrome (Table 1), and each mutation may have different clinical implications. Loss of function mutations in potassium channel genes produce decreases in outward repolarizing current and are responsible for LQT subtypes 1, 2, 5, 6, and 7. HERG and KCNE2 (MiRP1) genes