
4.Drug Biotransformation Maria Almira Correia,PhD INTRODUCTION Humans are exposed daily to a wide variety of foreign compounds called xenobiotics%4substances absorbed across the lungs or skin or,more commonly, ingested either unintentionally as compounds present in food and drink or deliberately as drugs for therapeutic or "recreational"purposes.Exposure to environmental xenobiotics may be inadvertent and accidental or%when they are present as components of air,water,and foodinescapable.Some xenobiotics are innocuous, but many can provoke biologic responses.Such biologic responses often depend on conversion of the absorbed substance into an active metabolite.The discussion that follows is applicable to xenobiotics in general(including drugs)and to some extent to endogenous compounds. WHY IS DRUG BIOTRANSFORMATION NECESSARY? Renal excretion plays a pivotal role in terminating the biologic activity of some drugs, particularly those that have small molecular volumes or possess polar characteristics such as functional groups that are fully ionized at physiologic pH.However,many drugs do not possess such physicochemical properties.Pharmacologically active organic molecules tend to be lipophilic and remain unionized or only partially ionized at physiologic pH;these are readily reabsorbed from the glomerular filtrate in the nephron.Certain lipophilic compounds are often strongly bound to plasma proteins and may not be readily filtered at the glomerulus.Consequently,most drugs would have a prolonged duration of action if termination of their action depended solely on renal excretion. An alternative process that can lead to the termination or alteration of biologic activity is metabolism.In general,lipophilic xenobiotics are transformed to more polar and hence more readily excreted products.The role metabolism plays in the inactivation of lipid-soluble drugs can be quite dramatic.For example,lipophilic barbiturates such as thiopental and pentobarbital would have extremely long half-lives if it were not for their metabolic conversion to more water-soluble compounds. Metabolic products are often less pharmacodynamically active than the parent drug and may even be inactive.However,some biotransformation products have enhanced activity or toxic properties.It is noteworthy that the synthesis of endogenous substrates such as steroid hormones,cholesterol,active vitamin D congeners,and bile acids involves many pathways catalyzed by enzymes associated with the metabolism of xenobiotics.Finally,drug-metabolizing enzymes have been exploited in the design of pharmacologically inactive prodrugs that are converted to active molecules in the body
4. Drug Biotransformation ¾ Maria Almira Correia, PhD INTRODUCTION Humans are exposed daily to a wide variety of foreign compounds called xenobiotics¾substances absorbed across the lungs or skin or, more commonly, ingested either unintentionally as compounds present in food and drink or deliberately as drugs for therapeutic or "recreational" purposes. Exposure to environmental xenobiotics may be inadvertent and accidental or¾when they are present as components of air, water, and food¾inescapable. Some xenobiotics are innocuous, but many can provoke biologic responses. Such biologic responses often depend on conversion of the absorbed substance into an active metabolite. The discussion that follows is applicable to xenobiotics in general (including drugs) and to some extent to endogenous compounds. WHY IS DRUG BIOTRANSFORMATION NECESSARY? Renal excretion plays a pivotal role in terminating the biologic activity of some drugs, particularly those that have small molecular volumes or possess polar characteristics such as functional groups that are fully ionized at physiologic pH. However, many drugs do not possess such physicochemical properties. Pharmacologically active organic molecules tend to be lipophilic and remain unionized or only partially ionized at physiologic pH; these are readily reabsorbed from the glomerular filtrate in the nephron. Certain lipophilic compounds are often strongly bound to plasma proteins and may not be readily filtered at the glomerulus. Consequently, most drugs would have a prolonged duration of action if termination of their action depended solely on renal excretion. An alternative process that can lead to the termination or alteration of biologic activity is metabolism. In general, lipophilic xenobiotics are transformed to more polar and hence more readily excreted products. The role metabolism plays in the inactivation of lipid-soluble drugs can be quite dramatic. For example, lipophilic barbiturates such as thiopental and pentobarbital would have extremely long half-lives if it were not for their metabolic conversion to more water-soluble compounds. Metabolic products are often less pharmacodynamically active than the parent drug and may even be inactive. However, some biotransformation products have enhanced activity or toxic properties. It is noteworthy that the synthesis of endogenous substrates such as steroid hormones, cholesterol, active vitamin D congeners, and bile acids involves many pathways catalyzed by enzymes associated with the metabolism of xenobiotics. Finally, drug-metabolizing enzymes have been exploited in the design of pharmacologically inactive prodrugs that are converted to active molecules in the body

THE ROLE OF BIOTRANSFORMATION IN DRUG DISPOSITION Most metabolic biotransformations occur at some point between absorption of the drug into the general circulation and its renal elimination.A few transformations occur in the intestinal lumen or intestinal wall.In general,all of these reactions can be assigned to one of two major categories called phase I and phase II reactions Figure 4-1). Phase I reactions usually convert the parent drug to a more polar metabolite by introducing or unmasking a functional group(-OH,-NH2,-SH).Often these metabolites are inactive,although in some instances activity is only modified or even enhanced. If phase I metabolites are sufficiently polar,they may be readily excreted.However, many phase I products are not eliminated rapidly and undergo a subsequent reaction in which an endogenous substrate such as glucuronic acid,sulfuric acid,acetic acid, or an amino acid combines with the newly incorporated functional group to form a highly polar conjugate.Such conjugation or synthetic reactions are the hallmarks of phase II metabolism.A great variety of drugs undergo these sequential biotransformation reactions,although in some instances the parent drug may already possess a functional group that may form a conjugate directly.For example,the hydrazide moiety of isoniazid is known to form an N-acetyl conjugate in a phase II reaction.This conjugate is then a substrate for a phase I type reaction,namely, hydrolysis to isonicotinic acid(Figure 4-2).Thus,phase II reactions may actually precede phase I reactions. ABSORPTION METABOLISM ELIMINATION Phase l Phase ll Drug Conjugate Drug metabolite with modified activity Conjugate Drug Inactive drug metabolite Conjugate Drug Lipophilic Hydrophilic
THE ROLE OF BIOTRANSFORMATION IN DRUG DISPOSITION Most metabolic biotransformations occur at some point between absorption of the drug into the general circulation and its renal elimination. A few transformations occur in the intestinal lumen or intestinal wall. In general, all of these reactions can be assigned to one of two major categories called phase I and phase II reactions ( Figure 4-1). Phase I reactions usually convert the parent drug to a more polar metabolite by introducing or unmasking a functional group (-OH, -NH2, -SH). Often these metabolites are inactive, although in some instances activity is only modified or even enhanced. If phase I metabolites are sufficiently polar, they may be readily excreted. However, many phase I products are not eliminated rapidly and undergo a subsequent reaction in which an endogenous substrate such as glucuronic acid, sulfuric acid, acetic acid, or an amino acid combines with the newly incorporated functional group to form a highly polar conjugate. Such conjugation or synthetic reactions are the hallmarks of phase II metabolism. A great variety of drugs undergo these sequential biotransformation reactions, although in some instances the parent drug may already possess a functional group that may form a conjugate directly. For example, the hydrazide moiety of isoniazid is known to form an N-acetyl conjugate in a phase II reaction. This conjugate is then a substrate for a phase I type reaction, namely, hydrolysis to isonicotinic acid (Figure 4-2). Thus, phase II reactions may actually precede phase I reactions

0 H C-N一NH2 (INH) Phase ll(acetylation) 0 H H 0 C-N一N-C- CH3 (N-acetyl INH) Phase I(hydrolysis) 0 0H C- OH +CH3-C-N-NH2(acetylhydrazine) Acetylation of Isonicotinic acid macromolecules (proteins) Hepatotoxicity Figure 4-1.Phase I and phase II reactions,and direct elimination,in drug biodisposition.Phase II reactions may also precede phase I reactions. Figure 4-2.Phase II activation of isoniazid(INH)to a hepatotoxic metabolite. WHERE DO DRUG BIOTRANSFORMATIONS OCCUR? Although every tissue has some ability to metabolize drugs,the liver is the principal organ of drug metabolism.Other tissues that display considerable activity include the gastrointestinal tract,the lungs,the skin,and the kidneys.Following oral administration,many drugs(eg,isoproterenol,meperidine,pentazocine,morphine) are absorbed intact from the small intestine and transported first via the portal system to the liver,where they undergo extensive metabolism.This process is called the first-pass effect(see Chapter 3).Some orally administered drugs (eg,clonazepam, chlorpromazine,cyclosporine)are more extensively metabolized in the intestine than in the liver,whereas others (eg,midazolam)undergo significant(50%)intestinal metabolism.Thus,intestinal metabolism can contribute to the overall first-pass effect, and individuals with compromised liver function may increasingly rely on such intestinal metabolism for drug elimination.First-pass effects may so greatly limit the bioavailability of orally administered drugs (eg,lidocaine)that alternative routes of administration must be used to achieve therapeutically effective blood levels. Furthermore,the lower gut harbors intestinal microorganisms that are capable of
Figure 4-1. Phase I and phase II reactions, and direct elimination, in drug biodisposition. Phase II reactions may also precede phase I reactions. Figure 4-2. Phase II activation of isoniazid (INH) to a hepatotoxic metabolite. WHERE DO DRUG BIOTRANSFORMATIONS OCCUR? Although every tissue has some ability to metabolize drugs, the liver is the principal organ of drug metabolism. Other tissues that display considerable activity include the gastrointestinal tract, the lungs, the skin, and the kidneys. Following oral administration, many drugs (eg, isoproterenol, meperidine, pentazocine, morphine) are absorbed intact from the small intestine and transported first via the portal system to the liver, where they undergo extensive metabolism. This process is called the first-pass effect (see Chapter 3). Some orally administered drugs (eg, clonazepam, chlorpromazine, cyclosporine) are more extensively metabolized in the intestine than in the liver, whereas others (eg, midazolam) undergo significant (50%) intestinal metabolism. Thus, intestinal metabolism can contribute to the overall first-pass effect, and individuals with compromised liver function may increasingly rely on such intestinal metabolism for drug elimination. First-pass effects may so greatly limit the bioavailability of orally administered drugs (eg, lidocaine) that alternative routes of administration must be used to achieve therapeutically effective blood levels. Furthermore, the lower gut harbors intestinal microorganisms that are capable of

many biotransformation reactions.In addition,drugs may be metabolized by gastric acid(eg,penicillin),by digestive enzymes(eg,polypeptides such as insulin),or by enzymes in the wall of the intestine (eg,sympathomimetic catecholamines) Although drug biotransformation in vivo can occur by spontaneous,noncatalyzed chemical reactions,the vast majority of transformations are catalyzed by specific cellular enzymes.At the subcellular level,these enzymes may be located in the endoplasmic reticulum (ER).mitochondria.cytosol,lysosomes.or even the nuclear envelope or plasma membrane. MICROSOMAL MIXED FUNCTION OXIDASE SYSTEM PHASE I REACTIONS Introduction Many drug-metabolizing enzymes are located in the lipophilic ER membranes of the liver and other tissues.When these lamellar membranes are isolated by homogenization and fractionation of the cell,they re-form into vesicles called microsomes.Microsomes retain most of the morphologic and functional characteristics of the intact membranes,including the rough and smooth surface features of the rough(ribosome-studded)and smooth(no ribosomes)ER.Whereas the rough microsomes tend to be dedicated to protein synthesis,the smooth microsomes are relatively rich in enzymes responsible for oxidative drug metabolism.In particular, they contain the important class of enzymes known as the mixed function oxidases (MFOs),or monooxygenases.The activity of these enzymes requires both a reducing agent (nicotinamide adenine dinucleotide phosphate [NADPH])and molecular oxygen;in a typical reaction,one molecule of oxygen is consumed(reduced)per substrate molecule,with one oxygen atom appearing in the product and the other in the form of water. In this oxidation-reduction process,two microsomal enzymes play a key role.The first of these is a flavoprotein,NADPH-cytochrome P450 reductase.One mole of this enzyme contains 1 mol each of flavin mononucleotide (FMN)and flavin adenine dinucleotide(FAD).The second microsomal enzyme is a hemoprotein called cytochrome P450,which serves as the terminal oxidase.In fact,the microsomal membrane harbors multiple forms of this hemoprotein,and this multiplicity is increased by repeated administration of exogenous chemicals(see below).The name cytochrome P450(abbreviated as CYP or P450)is derived from the spectral properties of this hemoprotein.In its reduced (ferrous)form,it binds carbon monoxide to give a complex that absorbs light maximally at 450 nm.The relative abundance of P450s,compared with that of the reductase in the liver,contributes to making P450 heme reduction a rate-limiting step in hepatic drug oxidations. Microsomal drug oxidations require P450,P450 reductase,NADPH,and molecular
many biotransformation reactions. In addition, drugs may be metabolized by gastric acid (eg, penicillin), by digestive enzymes (eg, polypeptides such as insulin), or by enzymes in the wall of the intestine (eg, sympathomimetic catecholamines). Although drug biotransformation in vivo can occur by spontaneous, noncatalyzed chemical reactions, the vast majority of transformations are catalyzed by specific cellular enzymes. At the subcellular level, these enzymes may be located in the endoplasmic reticulum (ER), mitochondria, cytosol, lysosomes, or even the nuclear envelope or plasma membrane. MICROSOMAL MIXED FUNCTION OXIDASE SYSTEM & PHASE I REACTIONS Introduction Many drug-metabolizing enzymes are located in the lipophilic ER membranes of the liver and other tissues. When these lamellar membranes are isolated by homogenization and fractionation of the cell, they re-form into vesicles called microsomes. Microsomes retain most of the morphologic and functional characteristics of the intact membranes, including the rough and smooth surface features of the rough (ribosome-studded) and smooth (no ribosomes) ER. Whereas the rough microsomes tend to be dedicated to protein synthesis, the smooth microsomes are relatively rich in enzymes responsible for oxidative drug metabolism. In particular, they contain the important class of enzymes known as the mixed function oxidases (MFOs), or monooxygenases. The activity of these enzymes requires both a reducing agent (nicotinamide adenine dinucleotide phosphate [NADPH]) and molecular oxygen; in a typical reaction, one molecule of oxygen is consumed (reduced) per substrate molecule, with one oxygen atom appearing in the product and the other in the form of water. In this oxidation-reduction process, two microsomal enzymes play a key role. The first of these is a flavoprotein, NADPH-cytochrome P450 reductase. One mole of this enzyme contains 1 mol each of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). The second microsomal enzyme is a hemoprotein called cytochrome P450, which serves as the terminal oxidase. In fact, the microsomal membrane harbors multiple forms of this hemoprotein, and this multiplicity is increased by repeated administration of exogenous chemicals (see below). The name cytochrome P450 (abbreviated as CYP or P450) is derived from the spectral properties of this hemoprotein. In its reduced (ferrous) form, it binds carbon monoxide to give a complex that absorbs light maximally at 450 nm. The relative abundance of P450s, compared with that of the reductase in the liver, contributes to making P450 heme reduction a rate-limiting step in hepatic drug oxidations. Microsomal drug oxidations require P450, P450 reductase, NADPH, and molecular
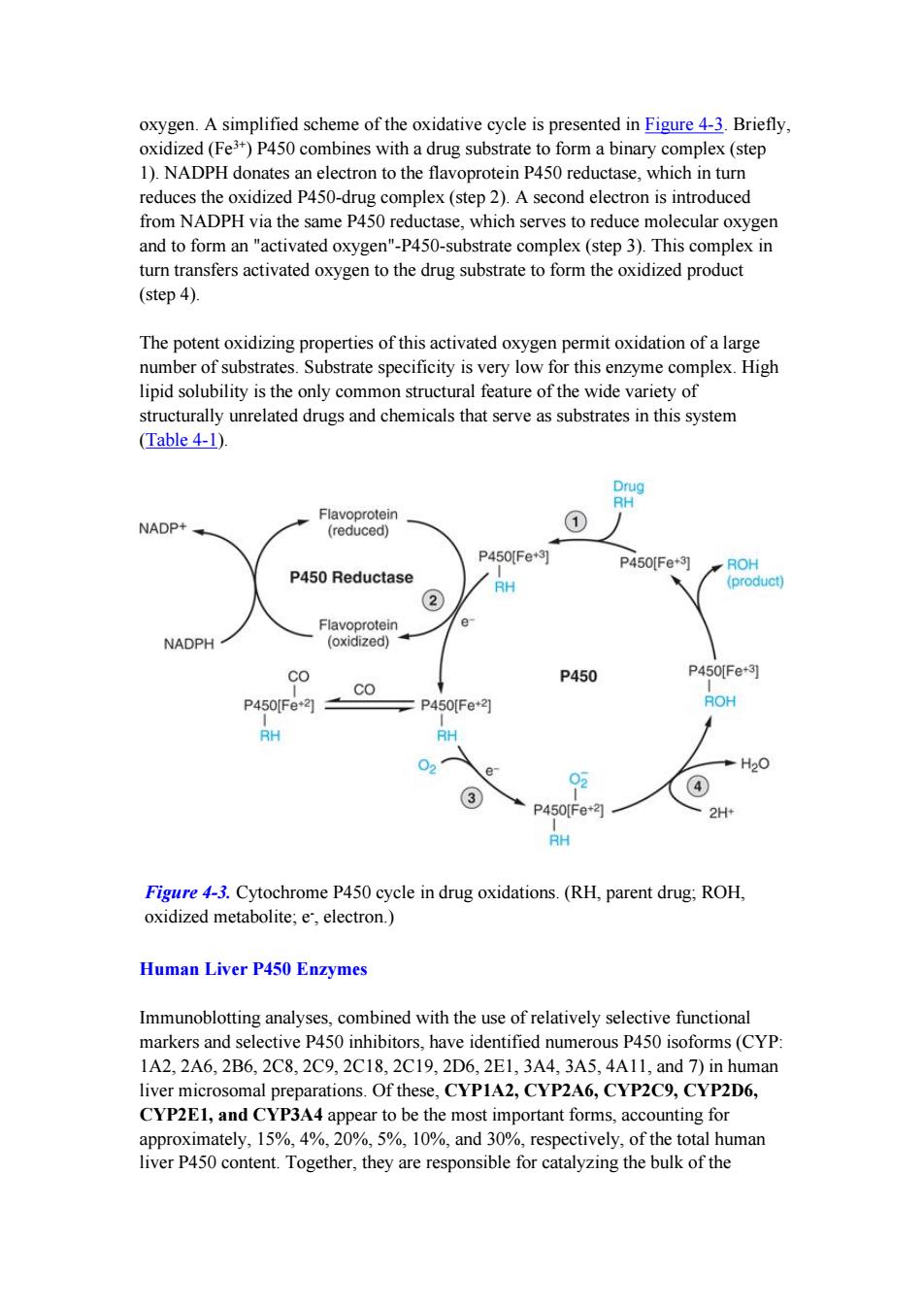
oxygen.A simplified scheme of the oxidative cycle is presented in Figure 4-3.Briefly, oxidized(Fe3+)P450 combines with a drug substrate to form a binary complex(step 1).NADPH donates an electron to the flavoprotein P450 reductase,which in turn reduces the oxidized P450-drug complex(step 2).A second electron is introduced from NADPH via the same P450 reductase,which serves to reduce molecular oxygen and to form an "activated oxygen"-P450-substrate complex (step 3).This complex in turn transfers activated oxygen to the drug substrate to form the oxidized product (step 4). The potent oxidizing properties of this activated oxygen permit oxidation of a large number of substrates.Substrate specificity is very low for this enzyme complex.High lipid solubility is the only common structural feature of the wide variety of structurally unrelated drugs and chemicals that serve as substrates in this system (Table 4-1). Drug RH Flavoprotein NADP+ (reduced) P450Fe+3] P450[Fe+3] ROH P450 Reductase RH (product) ② Flavoprotein e NADPH (oxidized) co P450 P450Fe+3 CO P450[Fe21 P450Fe+2 ROH RH RH H20 02 4 P450[Fe*3 2H+ RH Figure 4-3.Cytochrome P450 cycle in drug oxidations.(RH,parent drug;ROH, oxidized metabolite;e,electron.) Human Liver P450 Enzymes Immunoblotting analyses,combined with the use of relatively selective functional markers and selective P450 inhibitors,have identified numerous P450 isoforms(CYP: 1A2,2A6,2B6,2C8,2C9,2C18,2C19,2D6,2E1,3A4,3A5,4A11,and7)in human liver microsomal preparations.Of these,CYP1A2,CYP2A6,CYP2C9,CYP2D6, CYP2E1,and CYP3A4 appear to be the most important forms,accounting for approximately,15%,4%,20%,5%,10%,and 30%,respectively,of the total human liver P450 content.Together,they are responsible for catalyzing the bulk of the
oxygen. A simplified scheme of the oxidative cycle is presented in Figure 4-3. Briefly, oxidized (Fe 3+ ) P450 combines with a drug substrate to form a binary complex (step 1). NADPH donates an electron to the flavoprotein P450 reductase, which in turn reduces the oxidized P450-drug complex (step 2). A second electron is introduced from NADPH via the same P450 reductase, which serves to reduce molecular oxygen and to form an "activated oxygen"-P450-substrate complex (step 3). This complex in turn transfers activated oxygen to the drug substrate to form the oxidized product (step 4). The potent oxidizing properties of this activated oxygen permit oxidation of a large number of substrates. Substrate specificity is very low for this enzyme complex. High lipid solubility is the only common structural feature of the wide variety of structurally unrelated drugs and chemicals that serve as substrates in this system (Table 4-1). Figure 4-3. Cytochrome P450 cycle in drug oxidations. (RH, parent drug; ROH, oxidized metabolite; e -, electron.) Human Liver P450 Enzymes Immunoblotting analyses, combined with the use of relatively selective functional markers and selective P450 inhibitors, have identified numerous P450 isoforms (CYP: 1A2, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, 4A11, and 7) in human liver microsomal preparations. Of these, CYP1A2, CYP2A6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 appear to be the most important forms, accounting for approximately, 15%, 4%, 20%, 5%, 10%, and 30%, respectively, of the total human liver P450 content. Together, they are responsible for catalyzing the bulk of the

hepatic drug and xenobiotic metabolism(Table 4-2). It is noteworthy that CYP3A4 alone is responsible for the metabolism of over 50%of the clinically prescribed drugs metabolized by the liver.The involvement of individual P450s in the metabolism of a given drug may be screened in vitro by means of selective functional markers,selective chemical P450 inhibitors,and anti-P450 antibodies.In vivo,such screening may be accomplished by means of relatively selective noninvasive markers,which include breath tests or urinary analyses of specific metabolites after administration of a P450-selective substrate probe. Enzyme Induction Some of the chemically dissimilar P450 substrate drugs,on repeated administration, induce P450 by enhancing the rate of its synthesis or reducing its rate of degradation (Table 4-2).Induction results in an acceleration of substrate metabolism and usually in a decrease in the pharmacologic action of the inducer and also of coadministered drugs.However,in the case of drugs metabolically transformed to reactive metabolites,enzyme induction may exacerbate metabolite-mediated toxicity Various substrates induce P450 isoforms having different molecular masses and exhibiting different substrate specificities and immunochemical and spectral characteristics. Environmental pollutants are also capable of inducing P450 enzymes.As noted above, exposure to benzo[a]pyrene and other polycyclic aromatic hydrocarbons,which are present in tobacco smoke,charcoal-broiled meat,and other organic pyrolysis products, is known to induce CYPIA enzymes and to alter the rates of drug metabolism.Other environmental chemicals known to induce specific P450s include the polychlorinated biphenyls(PCBs),which were once used widely in industry as insulating materials and plasticizers,and 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin,TCDD),a trace byproduct of the chemical synthesis of the defoliant 2,4,5-T(see Chapter 57). Increased P450 synthesis requires enhanced transcription and translation.A cytoplasmic receptor(termed AhR)for polycyclic aromatic hydrocarbons(eg. benzo[a]pyrene,dioxin)has been identified,and the translocation of the inducer-receptor complex into the nucleus and subsequent activation of regulatory elements of genes have been documented.A pregnane X receptor(PXR),a member of the steroid-retinoid-thyroid hormone receptor family,has recently been shown to mediate CYP3A induction by various chemicals(dexamethasone,rifampin)in the liver and intestinal mucosa.A similar receptor,the constitutive androstane receptor (CAR)has been identified for the phenobarbital class of inducers P450 enzymes may also be induced by substrate stabilization,ie,decreased
hepatic drug and xenobiotic metabolism (Table 4-2). It is noteworthy that CYP3A4 alone is responsible for the metabolism of over 50% of the clinically prescribed drugs metabolized by the liver. The involvement of individual P450s in the metabolism of a given drug may be screened in vitro by means of selective functional markers, selective chemical P450 inhibitors, and anti-P450 antibodies. In vivo, such screening may be accomplished by means of relatively selective noninvasive markers, which include breath tests or urinary analyses of specific metabolites after administration of a P450-selective substrate probe. Enzyme Induction Some of the chemically dissimilar P450 substrate drugs, on repeated administration, induce P450 by enhancing the rate of its synthesis or reducing its rate of degradation (Table 4-2). Induction results in an acceleration of substrate metabolism and usually in a decrease in the pharmacologic action of the inducer and also of coadministered drugs. However, in the case of drugs metabolically transformed to reactive metabolites, enzyme induction may exacerbate metabolite-mediated toxicity. Various substrates induce P450 isoforms having different molecular masses and exhibiting different substrate specificities and immunochemical and spectral characteristics. Environmental pollutants are also capable of inducing P450 enzymes. As noted above, exposure to benzo[a]pyrene and other polycyclic aromatic hydrocarbons, which are present in tobacco smoke, charcoal-broiled meat, and other organic pyrolysis products, is known to induce CYP1A enzymes and to alter the rates of drug metabolism. Other environmental chemicals known to induce specific P450s include the polychlorinated biphenyls (PCBs), which were once used widely in industry as insulating materials and plasticizers, and 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin, TCDD), a trace byproduct of the chemical synthesis of the defoliant 2,4,5-T (see Chapter 57). Increased P450 synthesis requires enhanced transcription and translation. A cytoplasmic receptor (termed AhR) for polycyclic aromatic hydrocarbons (eg, benzo[a]pyrene, dioxin) has been identified, and the translocation of the inducer-receptor complex into the nucleus and subsequent activation of regulatory elements of genes have been documented. A pregnane X receptor (PXR), a member of the steroid-retinoid-thyroid hormone receptor family, has recently been shown to mediate CYP3A induction by various chemicals (dexamethasone, rifampin) in the liver and intestinal mucosa. A similar receptor, the constitutive androstane receptor (CAR) has been identified for the phenobarbital class of inducers. P450 enzymes may also be induced by substrate stabilization, ie, decreased

degradation,as is the case with troleandomycin-or clotrimazole-mediated induction of CYP3A enzymes and the ethanol-mediated induction of CYP2E1 Enzyme Inhibition Certain drug substrates inhibit cytochrome P450 enzyme activity (Table 4-2) Imidazole-containing drugs such as cimetidine and ketoconazole bind tightly to the P450 heme iron and effectively reduce the metabolism of endogenous substrates(eg testosterone)or other coadministered drugs through competitive inhibition.However, macrolide antibiotics such as troleandomycin,erythromycin,and erythromycin derivatives are metabolized,apparently by CYP3A,to metabolites that complex the cytochrome P450 heme-iron and render it catalytically inactive.Another compound that acts through this mechanism is the inhibitor proadifen(SKF-525-A,used in research),which binds tightly to the heme iron and quasi-irreversibly inactivates the enzyme,thereby inhibiting the metabolism of potential substrates Some substrates irreversibly inhibit P450s via covalent interaction of a metabolically generated reactive intermediate that may react with the P450 apoprotein or heme moiety or even cause the heme to fragment and irreversibly modify the apoprotein. The antibiotic chloramphenicol is metabolized by CYP2B1 to a species that modifies its protein and thus also inactivates the enzyme.A growing list of such suicide inhibitors%inactivators that attack the heme or the protein moietyincludes certain steroids(ethinyl estradiol,norethindrone,and spironolactone);fluroxene;allobarbital; the analgesic sedatives allylisopropylacetylurea,diethylpentenamide,and ethchlorvynol;carbon disulfide;grapefruit furanocoumarins;deprenyl;phencyclidine; ticlopidine and clopidogrel;ritonavir,and propylthiouracil.On the other hand,the barbiturate secobarbital is found to inactivate CYP2BI by modification of both its heme and protein moieties.Other metabolically activated drugs whose P450 inactivation mechanism is not fully elucidated include mifepristone(RU-486), troglitazone,raloxifene,and tamoxifen. PHASE II REACTIONS Parent drugs or their phase I metabolites that contain suitable chemical groups often undergo coupling or conjugation reactions with an endogenous substance to yield drug conjugates(Table 4-3).In general,conjugates are polar molecules that are readily excreted and often inactive.Conjugate formation involves high-energy intermediates and specific transfer enzymes.Such enzymes (transferases)may be located in microsomes or in the cytosol.They catalyze the coupling of an activated endogenous substance(such as the uridine 5c-diphosphate [UDP]derivative of glucuronic acid)with a drug (or endogenous compound),or of an activated drug(such as the S-CoA derivative of benzoic acid)with an endogenous substrate.Because the endogenous substrates originate in the diet,nutrition plays a critical role in the regulation of drug conjugations
degradation, as is the case with troleandomycin- or clotrimazole-mediated induction of CYP3A enzymes and the ethanol-mediated induction of CYP2E1. Enzyme Inhibition Certain drug substrates inhibit cytochrome P450 enzyme activity (Table 4-2). Imidazole-containing drugs such as cimetidine and ketoconazole bind tightly to the P450 heme iron and effectively reduce the metabolism of endogenous substrates (eg, testosterone) or other coadministered drugs through competitive inhibition. However, macrolide antibiotics such as troleandomycin, erythromycin, and erythromycin derivatives are metabolized, apparently by CYP3A, to metabolites that complex the cytochrome P450 heme-iron and render it catalytically inactive. Another compound that acts through this mechanism is the inhibitor proadifen (SKF-525-A, used in research), which binds tightly to the heme iron and quasi-irreversibly inactivates the enzyme, thereby inhibiting the metabolism of potential substrates. Some substrates irreversibly inhibit P450s via covalent interaction of a metabolically generated reactive intermediate that may react with the P450 apoprotein or heme moiety or even cause the heme to fragment and irreversibly modify the apoprotein. The antibiotic chloramphenicol is metabolized by CYP2B1 to a species that modifies its protein and thus also inactivates the enzyme. A growing list of such suicide inhibitors¾inactivators that attack the heme or the protein moiety¾includes certain steroids (ethinyl estradiol, norethindrone, and spironolactone); fluroxene; allobarbital; the analgesic sedatives allylisopropylacetylurea, diethylpentenamide, and ethchlorvynol; carbon disulfide; grapefruit furanocoumarins; deprenyl; phencyclidine; ticlopidine and clopidogrel; ritonavir, and propylthiouracil. On the other hand, the barbiturate secobarbital is found to inactivate CYP2B1 by modification of both its heme and protein moieties. Other metabolically activated drugs whose P450 inactivation mechanism is not fully elucidated include mifepristone (RU-486), troglitazone, raloxifene, and tamoxifen. PHASE II REACTIONS Parent drugs or their phase I metabolites that contain suitable chemical groups often undergo coupling or conjugation reactions with an endogenous substance to yield drug conjugates (Table 4-3). In general, conjugates are polar molecules that are readily excreted and often inactive. Conjugate formation involves high-energy intermediates and specific transfer enzymes. Such enzymes (transferases) may be located in microsomes or in the cytosol. They catalyze the coupling of an activated endogenous substance (such as the uridine 5¢-diphosphate [UDP] derivative of glucuronic acid) with a drug (or endogenous compound), or of an activated drug (such as the S-CoA derivative of benzoic acid) with an endogenous substrate. Because the endogenous substrates originate in the diet, nutrition plays a critical role in the regulation of drug conjugations

Drug conjugations were once believed to represent terminal inactivation events and as such have been viewed as "true detoxification"reactions.However,this concept must be modified.because it is now known that certain conjugation reactions (acyl glucuronidation of nonsteroidal anti-inflammatory drugs,O-sulfation of N-hydroxyacetylaminofluorene,and N-acetylation of isoniazid)may lead to the formation of reactive species responsible for the toxicity of the drugs.Furthermore, sulfation is known to activate the orally active prodrug minoxidil into a very efficacious vasodilator and morphine-6-glucuronide is more potent than morphine itself. METABOLISM OF DRUGS TO TOXIC PRODUCTS Metabolism of drugs and other foreign chemicals may not always be an innocuous biochemical event leading to detoxification and elimination of the compound.Indeed, as noted above,several compounds have been shown to be metabolically transformed to reactive intermediates that are toxic to various organs.Such toxic reactions may not be apparent at low levels of exposure to parent compounds when alternative detoxification mechanisms are not yet overwhelmed or compromised and the availability of endogenous detoxifying cosubstrates(glutathione [GSH],glucuronic acid,sulfate)is not limited.However,when these resources are exhausted,the toxic pathway may prevail,resulting in overt organ toxicity or carcinogenesis.The number of specific examples of such drug-induced toxicity is expanding rapidly.An example is acetaminophen(paracetamol)-induced hepatotoxicity (Figure 4-4).This analgesic antipyretic drug is quite safe in therapeutic doses(1.2 g/d for an adult).It normally undergoes glucuronidation and sulfation to the corresponding conjugates,which together comprise 95%of the total excreted metabolites.The alternative P450-dependent GSH conjugation pathway accounts for the remaining 5%.When acetaminophen intake far exceeds therapeutic doses,the glucuronidation and sulfation pathways are saturated,and the P450-dependent pathway becomes increasingly important.Little or no hepatotoxicity results as long as GSH is available for conjugation.However,with time,hepatic GSH is depleted faster than it can be regenerated,and a reactive,toxic metabolite accumulates.In the absence of intracellular nucleophiles such as GSH,this reactive metabolite (N-acetylbenzoiminoquinone)reacts with nucleophilic groups of cellular proteins, resulting in hepatotoxicity. The chemical and toxicologic characterization of the electrophilic nature of the reactive acetaminophen metabolite has led to the development of effective antidotes%cysteamine and N-acetylcysteine.Administration of N-acetylcysteine(the safer of the two)within 8-16 hours following acetaminophen overdosage has been shown to protect victims from fulminant hepatotoxicity and death(see Chapter 59) Administration of GSH is not effective because it does not cross cell membranes readily
Drug conjugations were once believed to represent terminal inactivation events and as such have been viewed as "true detoxification" reactions. However, this concept must be modified, because it is now known that certain conjugation reactions (acyl glucuronidation of nonsteroidal anti-inflammatory drugs, O-sulfation of N-hydroxyacetylaminofluorene, and N-acetylation of isoniazid) may lead to the formation of reactive species responsible for the toxicity of the drugs. Furthermore, sulfation is known to activate the orally active prodrug minoxidil into a very efficacious vasodilator and morphine-6-glucuronide is more potent than morphine itself. METABOLISM OF DRUGS TO TOXIC PRODUCTS Metabolism of drugs and other foreign chemicals may not always be an innocuous biochemical event leading to detoxification and elimination of the compound. Indeed, as noted above, several compounds have been shown to be metabolically transformed to reactive intermediates that are toxic to various organs. Such toxic reactions may not be apparent at low levels of exposure to parent compounds when alternative detoxification mechanisms are not yet overwhelmed or compromised and the availability of endogenous detoxifying cosubstrates (glutathione [GSH], glucuronic acid, sulfate) is not limited. However, when these resources are exhausted, the toxic pathway may prevail, resulting in overt organ toxicity or carcinogenesis. The number of specific examples of such drug-induced toxicity is expanding rapidly. An example is acetaminophen (paracetamol)-induced hepatotoxicity (Figure 4-4). This analgesic antipyretic drug is quite safe in therapeutic doses (1.2 g/d for an adult). It normally undergoes glucuronidation and sulfation to the corresponding conjugates, which together comprise 95% of the total excreted metabolites. The alternative P450-dependent GSH conjugation pathway accounts for the remaining 5%. When acetaminophen intake far exceeds therapeutic doses, the glucuronidation and sulfation pathways are saturated, and the P450-dependent pathway becomes increasingly important. Little or no hepatotoxicity results as long as GSH is available for conjugation. However, with time, hepatic GSH is depleted faster than it can be regenerated, and a reactive, toxic metabolite accumulates. In the absence of intracellular nucleophiles such as GSH, this reactive metabolite (N-acetylbenzoiminoquinone) reacts with nucleophilic groups of cellular proteins, resulting in hepatotoxicity. The chemical and toxicologic characterization of the electrophilic nature of the reactive acetaminophen metabolite has led to the development of effective antidotes¾cysteamine and N-acetylcysteine. Administration of N-acetylcysteine (the safer of the two) within 8-16 hours following acetaminophen overdosage has been shown to protect victims from fulminant hepatotoxicity and death (see Chapter 59). Administration of GSH is not effective because it does not cross cell membranes readily

Acetaminophen NHCOCH3 Glucuronidation Sulfation COOH 0. OH UDP OH 0 ADP S03H OH CYP2E1 CYP3A4 OH NHCOCH3 HONCOCH3 NHCOCH3 COOH OH OH 0S03H OH Reactive Toxic Intermediates NCOCH3 Nontoxic Nontoxic glucuronide sulfate 0 Nucleophillic GSH-Conjugation Cell Macromolecules GSH (Protein-SH) NHCOCH3 NHCOCH3 SG S-Protein OH OH ↓ NHCOCH3 ↓ LIVER CELL DEATH SCH2CHNHCOCH3 OH COOH Mercapturic Acid Conjugate Figure 4-4.Metabolism of acetaminophen (top center)to hepatotoxic metabolites. (GSH,glutathione;SG,glutathione moiety). CLINICAL RELEVANCE OF DRUG METABOLISM Introduction The dose and frequency of administration required to achieve effective therapeutic blood and tissue levels vary in different patients because of individual differences in
Figure 4-4. Metabolism of acetaminophen (top center) to hepatotoxic metabolites. (GSH, glutathione; SG, glutathione moiety). CLINICAL RELEVANCE OF DRUG METABOLISM Introduction The dose and frequency of administration required to achieve effective therapeutic blood and tissue levels vary in different patients because of individual differences in

drug distribution and rates of drug metabolism and elimination.These differences are determined by genetic factors and nongenetic variables such as age,sex,liver size, liver function,circadian rhythm,body temperature,and nutritional and environmental factors such as concomitant exposure to inducers or inhibitors of drug metabolism. The discussion that follows summarizes the most important of these variables. Individual Differences Individual differences in metabolic rate depend on the nature of the drug itself.Thus, within the same population,steady-state plasma levels may reflect a 30-fold variation in the metabolism of one drug and only a two-fold variation in the metabolism of another. Genetic Factors Genetic factors that influence enzyme levels account for some of these differences. Succinylcholine,for example,is metabolized only half as rapidly in persons with genetically determined defects in pseudocholinesterase as in persons with normally functioning pseudocholinesterase.Analogous pharmacogenetic differences are seen in the acetylation of isoniazid and the hydroxylation of warfarin.The defect in slow acetylators (of isoniazid and similar amines)appears to be caused by the synthesis of less of the enzyme rather than of an abnormal form of it.Inherited as an autosomal recessive trait,the slow acetylator phenotype occurs in about 50%of blacks and whites in the USA,more frequently in Europeans living in high northern latitudes and much less commonly in Asians and Inuits(Eskimos).Similarly,genetically determined defects in the oxidative metabolism of debrisoquin,phenacetin,guanoxan, sparteine,phenformin,warfarin,and others have been reported(Table 4-4).The defects are apparently transmitted as autosomal recessive traits and may be expressed at any one of the multiple metabolic transformations that a chemical might undergo. Of the several recognized genetic varieties of drug metabolism polymorphisms,three have been particularly well characterized and afford some insight into possible underlying mechanisms.First is the debrisoquin-sparteine oxidation type of polymorphism,which apparently occurs in 3-10%of whites and is inherited as an autosomal recessive trait.In affected individuals,the CYP2D6-dependent oxidations of debrisoquin and other drugs(see Table 4-2;Figure 4-5)are impaired.These defects in oxidative drug metabolism are probably coin-herited.The precise molecular basis for the defect appears to be faulty expression of the P450 protein,resulting in little or no isoform-catalyzed drug metabolism.More recently,however,another polymorphic genotype has been reported that results in ultrarapid metabolism of relevant drugs due to the presence of 2D6 allelic variants with up to 13 gene copies in tandem.This genotype is most common in Ethiopians and Saudi Arabians,populations that display it in up to one third of individuals.As a result,these subjects require twofold to threefold higher daily doses of nortriptyline (a 2D6 substrate)to achieve therapeutic
drug distribution and rates of drug metabolism and elimination. These differences are determined by genetic factors and nongenetic variables such as age, sex, liver size, liver function, circadian rhythm, body temperature, and nutritional and environmental factors such as concomitant exposure to inducers or inhibitors of drug metabolism. The discussion that follows summarizes the most important of these variables. Individual Differences Individual differences in metabolic rate depend on the nature of the drug itself. Thus, within the same population, steady-state plasma levels may reflect a 30-fold variation in the metabolism of one drug and only a two-fold variation in the metabolism of another. Genetic Factors Genetic factors that influence enzyme levels account for some of these differences. Succinylcholine, for example, is metabolized only half as rapidly in persons with genetically determined defects in pseudocholinesterase as in persons with normally functioning pseudocholinesterase. Analogous pharmacogenetic differences are seen in the acetylation of isoniazid and the hydroxylation of warfarin. The defect in slow acetylators (of isoniazid and similar amines) appears to be caused by the synthesis of less of the enzyme rather than of an abnormal form of it. Inherited as an autosomal recessive trait, the slow acetylator phenotype occurs in about 50% of blacks and whites in the USA, more frequently in Europeans living in high northern latitudes, and much less commonly in Asians and Inuits (Eskimos). Similarly, genetically determined defects in the oxidative metabolism of debrisoquin, phenacetin, guanoxan, sparteine, phenformin, warfarin, and others have been reported (Table 4-4). The defects are apparently transmitted as autosomal recessive traits and may be expressed at any one of the multiple metabolic transformations that a chemical might undergo. Of the several recognized genetic varieties of drug metabolism polymorphisms, three have been particularly well characterized and afford some insight into possible underlying mechanisms. First is the debrisoquin-sparteine oxidation type of polymorphism, which apparently occurs in 3-10% of whites and is inherited as an autosomal recessive trait. In affected individuals, the CYP2D6-dependent oxidations of debrisoquin and other drugs (see Table 4-2; Figure 4-5) are impaired. These defects in oxidative drug metabolism are probably coin-herited. The precise molecular basis for the defect appears to be faulty expression of the P450 protein, resulting in little or no isoform-catalyzed drug metabolism. More recently, however, another polymorphic genotype has been reported that results in ultrarapid metabolism of relevant drugs due to the presence of 2D6 allelic variants with up to 13 gene copies in tandem. This genotype is most common in Ethiopians and Saudi Arabians, populations that display it in up to one third of individuals. As a result, these subjects require twofold to threefold higher daily doses of nortriptyline (a 2D6 substrate) to achieve therapeutic