
English Reading Materials Chapter 33:Agents That Affect Bone Mineral Homeostasis I.BASIC PHARMACOLOGY INTRODUCTION Calcium and phosphate,the major mineral constituents of bone,are also two of the most important minerals for general cellular function.Accordingly,the body has evolved a complex set of mechanisms by which calcium and phosphate homeostasis are carefully maintained(Figure 1).Approximately 98%of the 1-2 kg of calcium and 85%of the 1 kg of phosphorus in the human adult are found in bone,the principal reservoir for these minerals.These functions are dynamic,with constant remodeling of bone and ready exchange of bone mineral with that in the extracellular fluid.Bone also serves as the principal structural support for the body and provides the space for hematopoiesis.Thus,abnormalities in bone mineral homeostasis can lead not only to a wide variety of cellular dysfunctions (eg,tetany,coma,muscle weakness)but also to disturbances in structural support of the body (eg,osteoporosis with fractures)and loss of hematopoietic capacity (eg,infantile osteopetrosis). Calcium and phosphate enter the body from the intestine.The average American diet provides 600-1000 mg of calcium per day,of which approximately 100-250 mg is absorbed.This figure represents net absorption,because both absorption(principally in the duodenum and upper jejunum)and secretion(principally in the ileum)occur. The amount of phosphorus in the American diet is about the same as that of calcium. However,the efficiency of absorption(principally in the jejunum)is greater,ranging from 70%to 90%,depending on intake.In the steady state,renal excretion of calcium and phosphate balances intestinal absorption.In general,over 98%of filtered calcium and 85%of filtered phosphate is reabsorbed by the kidney.The movement of calcium and phosphate across the intestinal and renal epithelia is closely regulated.Intrinsic disease of the intestine (eg,nontropical sprue)or kidney (eg,chronic renal failure) disrupts bone mineral homeostasis. Two hormones serve as the principal regulators of calcium and phosphate homeostasis: the peptide parathyroid hormone(PTH)and the steroid vitamin D(Figure 2).Vitamin D is a prohormone rather than a true hormone,because it must be further metabolized to gain biologic activity.PTH stimulates the production of the active metabolite of vitamin D,1,25(OH)2D.1,25(OH)2D,on the other hand,suppresses the production of PTH.1,25(OH)2D stimulates the intestinal absorption of calcium and phosphate. 1,25(OH)2D and PTH promote both bone formation and resorption in part by stimulating the proliferation and differentiation of osteoblasts and osteoclasts.Both PTH and 1,25(OH)2D enhance renal retention of calcium,but PTH promotes renal
English Reading Materials Chapter 33: Agents That Affect Bone Mineral Homeostasis I. BASIC PHARMACOLOGY INTRODUCTION Calcium and phosphate, the major mineral constituents of bone, are also two of the most important minerals for general cellular function. Accordingly, the body has evolved a complex set of mechanisms by which calcium and phosphate homeostasis are carefully maintained (Figure 1). Approximately 98% of the 1-2 kg of calcium and 85% of the 1 kg of phosphorus in the human adult are found in bone, the principal reservoir for these minerals. These functions are dynamic, with constant remodeling of bone and ready exchange of bone mineral with that in the extracellular fluid. Bone also serves as the principal structural support for the body and provides the space for hematopoiesis. Thus, abnormalities in bone mineral homeostasis can lead not only to a wide variety of cellular dysfunctions (eg, tetany, coma, muscle weakness) but also to disturbances in structural support of the body (eg, osteoporosis with fractures) and loss of hematopoietic capacity (eg, infantile osteopetrosis). Calcium and phosphate enter the body from the intestine. The average American diet provides 600-1000 mg of calcium per day, of which approximately 100-250 mg is absorbed. This figure represents net absorption, because both absorption (principally in the duodenum and upper jejunum) and secretion (principally in the ileum) occur. The amount of phosphorus in the American diet is about the same as that of calcium. However, the efficiency of absorption (principally in the jejunum) is greater, ranging from 70% to 90%, depending on intake. In the steady state, renal excretion of calcium and phosphate balances intestinal absorption. In general, over 98% of filtered calcium and 85% of filtered phosphate is reabsorbed by the kidney. The movement of calcium and phosphate across the intestinal and renal epithelia is closely regulated. Intrinsic disease of the intestine (eg, nontropical sprue) or kidney (eg, chronic renal failure) disrupts bone mineral homeostasis. Two hormones serve as the principal regulators of calcium and phosphate homeostasis: the peptide parathyroid hormone (PTH) and the steroid vitamin D (Figure 2). Vitamin D is a prohormone rather than a true hormone, because it must be further metabolized to gain biologic activity. PTH stimulates the production of the active metabolite of vitamin D, 1,25(OH)2D. 1,25(OH)2D, on the other hand, suppresses the production of PTH. 1,25(OH)2D stimulates the intestinal absorption of calcium and phosphate. 1,25(OH)2D and PTH promote both bone formation and resorption in part by stimulating the proliferation and differentiation of osteoblasts and osteoclasts. Both PTH and 1,25(OH)2D enhance renal retention of calcium, but PTH promotes renal
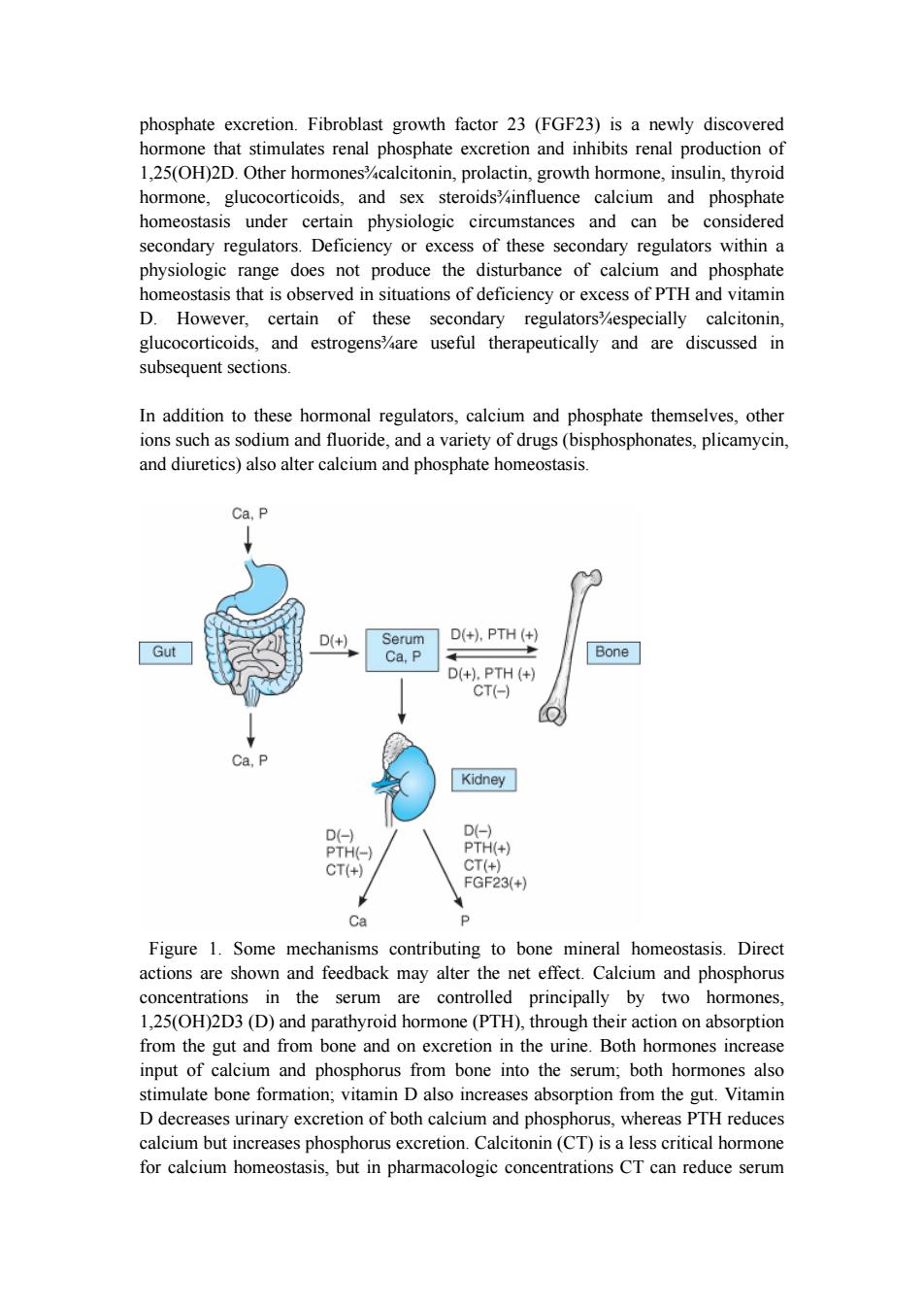
phosphate excretion.Fibroblast growth factor 23(FGF23)is a newly discovered hormone that stimulates renal phosphate excretion and inhibits renal production of 1,25(OH)2D.Other hormones%calcitonin,prolactin,growth hormone,insulin,thyroid hormone,glucocorticoids,and sex steroidsinfluence calcium and phosphate homeostasis under certain physiologic circumstances and can be considered secondary regulators.Deficiency or excess of these secondary regulators within a physiologic range does not produce the disturbance of calcium and phosphate homeostasis that is observed in situations of deficiency or excess of PTH and vitamin D.However,certain of these secondary regulators%especially calcitonin, glucocorticoids,and estrogens%are useful therapeutically and are discussed in subsequent sections. In addition to these hormonal regulators,calcium and phosphate themselves,other ions such as sodium and fluoride,and a variety of drugs(bisphosphonates,plicamycin, and diuretics)also alter calcium and phosphate homeostasis. Ca,P D(+) Serum D(+),PTH(+) Gut Ca,P Bone D(+),PTH(+) CT(-) Ca,P Kidney D(-) D(-) PTH(-) PTH(+) CT(+) CT(+) FGF23(+) Ca P Figure 1.Some mechanisms contributing to bone mineral homeostasis.Direct actions are shown and feedback may alter the net effect.Calcium and phosphorus concentrations in the serum are controlled principally by two hormones, 1,25(OH)2D3(D)and parathyroid hormone(PTH),through their action on absorption from the gut and from bone and on excretion in the urine.Both hormones increase input of calcium and phosphorus from bone into the serum;both hormones also stimulate bone formation;vitamin D also increases absorption from the gut.Vitamin D decreases urinary excretion of both calcium and phosphorus,whereas PTH reduces calcium but increases phosphorus excretion.Calcitonin(CT)is a less critical hormone for calcium homeostasis,but in pharmacologic concentrations CT can reduce serum
phosphate excretion. Fibroblast growth factor 23 (FGF23) is a newly discovered hormone that stimulates renal phosphate excretion and inhibits renal production of 1,25(OH)2D. Other hormones¾calcitonin, prolactin, growth hormone, insulin, thyroid hormone, glucocorticoids, and sex steroids¾influence calcium and phosphate homeostasis under certain physiologic circumstances and can be considered secondary regulators. Deficiency or excess of these secondary regulators within a physiologic range does not produce the disturbance of calcium and phosphate homeostasis that is observed in situations of deficiency or excess of PTH and vitamin D. However, certain of these secondary regulators¾especially calcitonin, glucocorticoids, and estrogens¾are useful therapeutically and are discussed in subsequent sections. In addition to these hormonal regulators, calcium and phosphate themselves, other ions such as sodium and fluoride, and a variety of drugs (bisphosphonates, plicamycin, and diuretics) also alter calcium and phosphate homeostasis. Figure 1. Some mechanisms contributing to bone mineral homeostasis. Direct actions are shown and feedback may alter the net effect. Calcium and phosphorus concentrations in the serum are controlled principally by two hormones, 1,25(OH)2D3 (D) and parathyroid hormone (PTH), through their action on absorption from the gut and from bone and on excretion in the urine. Both hormones increase input of calcium and phosphorus from bone into the serum; both hormones also stimulate bone formation; vitamin D also increases absorption from the gut. Vitamin D decreases urinary excretion of both calcium and phosphorus, whereas PTH reduces calcium but increases phosphorus excretion. Calcitonin (CT) is a less critical hormone for calcium homeostasis, but in pharmacologic concentrations CT can reduce serum
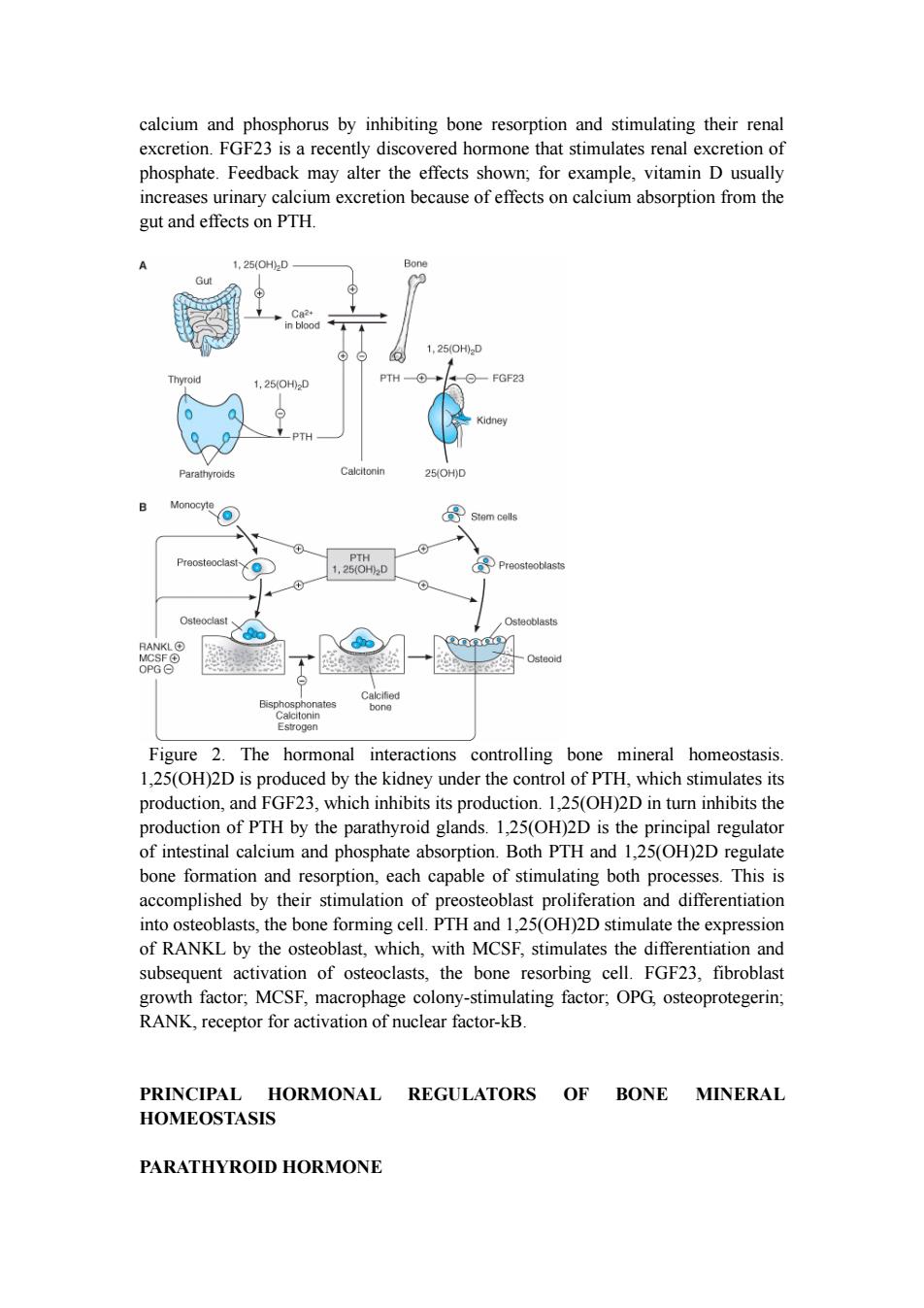
calcium and phosphorus by inhibiting bone resorption and stimulating their renal excretion.FGF23 is a recently discovered hormone that stimulates renal excretion of phosphate.Feedback may alter the effects shown;for example,vitamin D usually increases urinary calcium excretion because of effects on calcium absorption from the gut and effects on PTH. 1,25(OH2D Bon 1,25(0H0D Thy 1,25(0H02D PTH FGF23 Kidney PTH Parathyroids Calcitonin 25(OH)D Monocyte 导 Stem cells Preosteoclast PTH 1,25(0H02D Preosteoblasts Osteoclast Osteoblasts RANKLO Osteoid OPG⊙ Bisphosphonates Calcitonin Estrogen Figure 2.The hormonal interactions controlling bone mineral homeostasis. 1,25(OH)2D is produced by the kidney under the control of PTH,which stimulates its production,and FGF23,which inhibits its production.1,25(OH)2D in turn inhibits the production of PTH by the parathyroid glands.1,25(OH)2D is the principal regulator of intestinal calcium and phosphate absorption.Both PTH and 1,25(OH)2D regulate bone formation and resorption,each capable of stimulating both processes.This is accomplished by their stimulation of preosteoblast proliferation and differentiation into osteoblasts,the bone forming cell.PTH and 1,25(OH)2D stimulate the expression of RANKL by the osteoblast,which,with MCSF,stimulates the differentiation and subsequent activation of osteoclasts,the bone resorbing cell.FGF23,fibroblast growth factor;MCSF,macrophage colony-stimulating factor;OPG,osteoprotegerin; RANK,receptor for activation of nuclear factor-kB. PRINCIPAL HORMONAL REGULATORS OF BONE MINERAL HOMEOSTASIS PARATHYROID HORMONE
calcium and phosphorus by inhibiting bone resorption and stimulating their renal excretion. FGF23 is a recently discovered hormone that stimulates renal excretion of phosphate. Feedback may alter the effects shown; for example, vitamin D usually increases urinary calcium excretion because of effects on calcium absorption from the gut and effects on PTH. Figure 2. The hormonal interactions controlling bone mineral homeostasis. 1,25(OH)2D is produced by the kidney under the control of PTH, which stimulates its production, and FGF23, which inhibits its production. 1,25(OH)2D in turn inhibits the production of PTH by the parathyroid glands. 1,25(OH)2D is the principal regulator of intestinal calcium and phosphate absorption. Both PTH and 1,25(OH)2D regulate bone formation and resorption, each capable of stimulating both processes. This is accomplished by their stimulation of preosteoblast proliferation and differentiation into osteoblasts, the bone forming cell. PTH and 1,25(OH)2D stimulate the expression of RANKL by the osteoblast, which, with MCSF, stimulates the differentiation and subsequent activation of osteoclasts, the bone resorbing cell. FGF23, fibroblast growth factor; MCSF, macrophage colony-stimulating factor; OPG, osteoprotegerin; RANK, receptor for activation of nuclear factor-kB. PRINCIPAL HORMONAL REGULATORS OF BONE MINERAL HOMEOSTASIS PARATHYROID HORMONE

Parathyroid hormone(PTH)is a single-chain peptide hormone composed of 84 amino acids.It is produced in the parathyroid gland in a precursor form of 115 amino acids, the remaining 31 amino terminal amino acids being cleaved off before secretion. Within the gland is a calcium-sensitive protease capable of cleaving the intact hormone into fragments.Biologic activity resides in the amino terminal region such that synthetic 1-34 PTH is fully active.Loss of the first two amino terminal amino acids eliminates most biologic activity. The metabolic clearance of intact PTH is rapid,with a half-time of disappearance measured in minutes.Most of the clearance occurs in the liver and kidney.The biologically inactive carboxyl terminal fragments produced during metabolism of the intact hormone have a much lower clearance,especially in renal failure.This accounts in part for the very high PTH values often observed in the past in patients with renal failure when measured by radioimmunoassays directed against the carboxyl terminal region of the molecule.However,most PTH assays currently in use measure the intact hormone by a double antibody method,so that this circumstance is less frequently encountered in clinical practice.PTH regulates calcium and phosphate flux across cellular membranes in bone and kidney,resulting in increased serum calcium and decreased serum phosphate.In bone,PTH increases the activity and number of osteoclasts,the cells responsible for bone resorption.However,this stimulation of osteoclasts is not a direct effect.Rather,PTH acts on the osteoblast(the bone-forming cell)to induce a membrane-bound protein called RANK ligand(RANKL).This factor acts on osteoclasts and osteoclast precursors to increase both the numbers and the activity of osteoclasts.This action increases bone turnover or bone remodeling,a specific sequence of cellular events initiated by osteoclastic bone resorption and followed by osteoblastic bone formation.Although both bone resorption and bone formation are enhanced by PTH,the net effect of excess PTH is to increase bone resorption.PTH in low and intermittent doses increases bone formation without first stimulating bone resorption.This action appears to be indirect,involving other growth factors such as IGF-1.This has led to the recent approval of recombinant PTH 1-34 (teriparatide)for the treatment of osteoporosis.In the kidney,PTH increases the ability of the nephron to reabsorb calcium and magnesium but reduces its ability to reabsorb phosphate,amino acids,bicarbonate,sodium,chloride,and sulfate.Another important action of PTH on the kidney is its stimulation of 1,25-dihydroxyvitamin D (1,25[OH]2D)production. VITAMIN D Vitamin D is a secosteroid produced in the skin from 7-dehydrocholesterol under the influence of ultraviolet irradiation.Vitamin D is also found in certain foods and is used to supplement dairy products.Both the natural form(vitamin D3,cholecalciferol) and the plant-derived form(vitamin D2,ergocalciferol)are present in the diet.These forms differ in that ergocalciferol contains a double bond(C22-23)and an additional
Parathyroid hormone (PTH) is a single-chain peptide hormone composed of 84 amino acids. It is produced in the parathyroid gland in a precursor form of 115 amino acids, the remaining 31 amino terminal amino acids being cleaved off before secretion. Within the gland is a calcium-sensitive protease capable of cleaving the intact hormone into fragments. Biologic activity resides in the amino terminal region such that synthetic 1-34 PTH is fully active. Loss of the first two amino terminal amino acids eliminates most biologic activity. The metabolic clearance of intact PTH is rapid, with a half-time of disappearance measured in minutes. Most of the clearance occurs in the liver and kidney. The biologically inactive carboxyl terminal fragments produced during metabolism of the intact hormone have a much lower clearance, especially in renal failure. This accounts in part for the very high PTH values often observed in the past in patients with renal failure when measured by radioimmunoassays directed against the carboxyl terminal region of the molecule. However, most PTH assays currently in use measure the intact hormone by a double antibody method, so that this circumstance is less frequently encountered in clinical practice. PTH regulates calcium and phosphate flux across cellular membranes in bone and kidney, resulting in increased serum calcium and decreased serum phosphate. In bone, PTH increases the activity and number of osteoclasts, the cells responsible for bone resorption. However, this stimulation of osteoclasts is not a direct effect. Rather, PTH acts on the osteoblast (the bone-forming cell) to induce a membrane-bound protein called RANK ligand (RANKL). This factor acts on osteoclasts and osteoclast precursors to increase both the numbers and the activity of osteoclasts. This action increases bone turnover or bone remodeling, a specific sequence of cellular events initiated by osteoclastic bone resorption and followed by osteoblastic bone formation. Although both bone resorption and bone formation are enhanced by PTH, the net effect of excess PTH is to increase bone resorption. PTH in low and intermittent doses increases bone formation without first stimulating bone resorption. This action appears to be indirect, involving other growth factors such as IGF-1. This has led to the recent approval of recombinant PTH 1-34 (teriparatide) for the treatment of osteoporosis. In the kidney, PTH increases the ability of the nephron to reabsorb calcium and magnesium but reduces its ability to reabsorb phosphate, amino acids, bicarbonate, sodium, chloride, and sulfate. Another important action of PTH on the kidney is its stimulation of 1,25-dihydroxyvitamin D (1,25[OH]2D) production. VITAMIN D Vitamin D is a secosteroid produced in the skin from 7-dehydrocholesterol under the influence of ultraviolet irradiation. Vitamin D is also found in certain foods and is used to supplement dairy products. Both the natural form (vitamin D3, cholecalciferol) and the plant-derived form (vitamin D2, ergocalciferol) are present in the diet. These forms differ in that ergocalciferol contains a double bond (C22-23) and an additional

methyl group in the side chain(Figure 3).In humans,this difference apparently is of limited physiologic consequence (although ergocalciferol is less potent),and the following comments apply equally well to both forms of vitamin D. Vitamin D is a prohormone that serves as precursor to a number of biologically active metabolites (Figure 3).Vitamin D is first hydroxylated in the liver to form 25-hydroxyvitamin D(25[OH]D).This metabolite is further converted in the kidney to a number of other forms,the best-studied of which are 1,25-dihydroxyvitamin D (1,25[OH]2D)and 24,25-dihydroxyvitamin D (24,25[OH]2D).Of the natural metabolites,only vitamin D and 1,25(OH)2D (as calcitriol)are available for clinical use.Moreover,a number of analogs of 1,25(OH)2D are being synthesized to extend the usefulness of this metabolite to a variety of nonclassic conditions.Calcipotriene (calcipotriol),for example,is being used to treat psoriasis,a hyperproliferative skin disorder.Doxercalciferol and paricalcitol have recently been approved for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Other analogs are being investigated for the treatment of various malignancies.The regulation of vitamin D metabolism is complex,involving calcium,phosphate,and a variety of hormones,the most important of which is PTH,which stimulates the production of 1,25(OH)2D by the kidney. Vitamin D and its metabolites circulate in plasma tightly bound to a carrier protein, the vitamin D-binding protein.This a-globulin binds 25(OH)D and 24,25(OH)2D with comparable high affinity and vitamin D and 1,25(OH)2D with lower affinity.In normal subjects,the terminal half-life of injected calcifediol is 23 days,whereas in anephric subjects it is 42 days.The half-life of 24,25(OH)2D is probably similar. Tracer studies with vitamin D have shown a rapid clearance from the blood.The liver appears to be the principal organ for clearance.Excess vitamin D is stored in adipose tissue.The metabolic clearance of calcitriol in humans indicates a rapid turnover,with a terminal half-life measured in hours.Several of the 1,25(OH)2D analogs are bound poorly by the vitamin D-binding protein.As a result,their clearance is very rapid, with a terminal half-life measured in minutes.Such analogs have little of the hypercalcemic,hypercalciuric effects of calcitriol,an important aspect of their use for the management of conditions such as psoriasis and hyperparathyroidism The mechanism of action of the vitamin D metabolites remains under active investigation.However,calcitriol is well established as the most potent agent with respect to stimulation of intestinal calcium and phosphate transport and bone resorption.Calcitriol appears to act on the intestine both by induction of new protein synthesis (eg,calcium-binding protein)and by modulation of calcium flux across the brush border and basolateral membranes by a means that does not require new protein synthesis.The molecular action of calcitriol on bone has received less attention. However,like PTH,calcitriol can induce RANK ligand in osteoblasts and proteins such as osteocalcin,which may regulate the mineralization process.The metabolites 25(OH)D and 24,25(OH)2D are far less potent stimulators of intestinal calcium and
methyl group in the side chain (Figure 3). In humans, this difference apparently is of limited physiologic consequence (although ergocalciferol is less potent), and the following comments apply equally well to both forms of vitamin D. Vitamin D is a prohormone that serves as precursor to a number of biologically active metabolites (Figure 3). Vitamin D is first hydroxylated in the liver to form 25-hydroxyvitamin D (25[OH]D). This metabolite is further converted in the kidney to a number of other forms, the best-studied of which are 1,25-dihydroxyvitamin D (1,25[OH]2D) and 24,25-dihydroxyvitamin D (24,25[OH]2D). Of the natural metabolites, only vitamin D and 1,25(OH)2D (as calcitriol) are available for clinical use. Moreover, a number of analogs of 1,25(OH)2D are being synthesized to extend the usefulness of this metabolite to a variety of nonclassic conditions. Calcipotriene (calcipotriol), for example, is being used to treat psoriasis, a hyperproliferative skin disorder. Doxercalciferol and paricalcitol have recently been approved for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Other analogs are being investigated for the treatment of various malignancies. The regulation of vitamin D metabolism is complex, involving calcium, phosphate, and a variety of hormones, the most important of which is PTH, which stimulates the production of 1,25(OH)2D by the kidney. Vitamin D and its metabolites circulate in plasma tightly bound to a carrier protein, the vitamin D-binding protein. This a-globulin binds 25(OH)D and 24,25(OH)2D with comparable high affinity and vitamin D and 1,25(OH)2D with lower affinity. In normal subjects, the terminal half-life of injected calcifediol is 23 days, whereas in anephric subjects it is 42 days. The half-life of 24,25(OH)2D is probably similar. Tracer studies with vitamin D have shown a rapid clearance from the blood. The liver appears to be the principal organ for clearance. Excess vitamin D is stored in adipose tissue. The metabolic clearance of calcitriol in humans indicates a rapid turnover, with a terminal half-life measured in hours. Several of the 1,25(OH)2D analogs are bound poorly by the vitamin D-binding protein. As a result, their clearance is very rapid, with a terminal half-life measured in minutes. Such analogs have little of the hypercalcemic, hypercalciuric effects of calcitriol, an important aspect of their use for the management of conditions such as psoriasis and hyperparathyroidism. The mechanism of action of the vitamin D metabolites remains under active investigation. However, calcitriol is well established as the most potent agent with respect to stimulation of intestinal calcium and phosphate transport and bone resorption. Calcitriol appears to act on the intestine both by induction of new protein synthesis (eg, calcium-binding protein) and by modulation of calcium flux across the brush border and basolateral membranes by a means that does not require new protein synthesis. The molecular action of calcitriol on bone has received less attention. However, like PTH, calcitriol can induce RANK ligand in osteoblasts and proteins such as osteocalcin, which may regulate the mineralization process. The metabolites 25(OH)D and 24,25(OH)2D are far less potent stimulators of intestinal calcium and
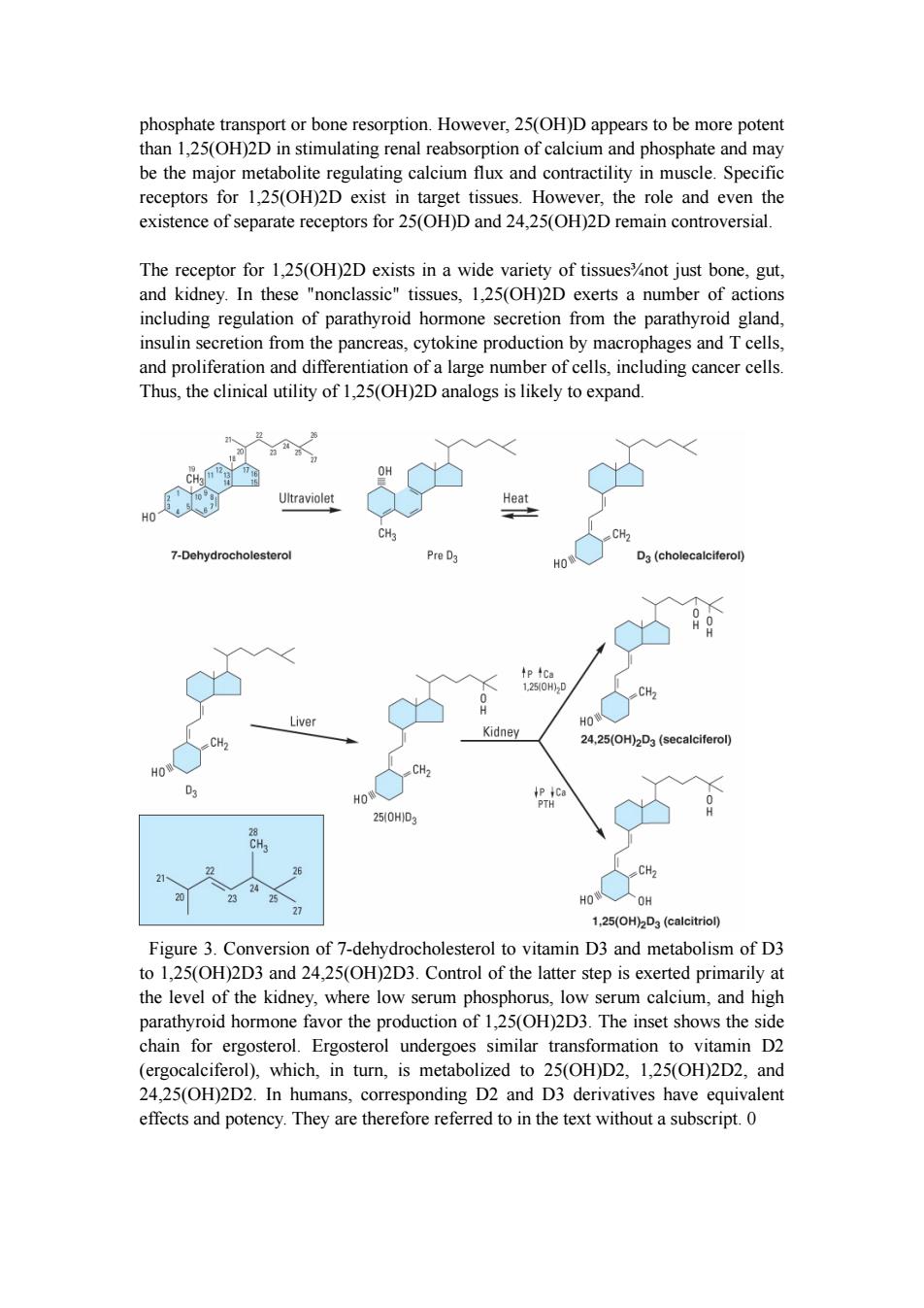
phosphate transport or bone resorption.However,25(OH)D appears to be more potent than 1,25(OH)2D in stimulating renal reabsorption of calcium and phosphate and may be the major metabolite regulating calcium flux and contractility in muscle.Specific receptors for 1,25(OH)2D exist in target tissues.However,the role and even the existence of separate receptors for 25(OH)D and 24,25(OH)2D remain controversial. The receptor for 1,25(OH)2D exists in a wide variety of tissues%not just bone,gut, and kidney.In these "nonclassic"tissues,1,25(OH)2D exerts a number of actions including regulation of parathyroid hormone secretion from the parathyroid gland, insulin secretion from the pancreas,cytokine production by macrophages and T cells, and proliferation and differentiation of a large number of cells,including cancer cells. Thus,the clinical utility of 1,25(OH)2D analogs is likely to expand 10 Ultraviolet Heat CH3 CH2 7-Dehydrocholesterol Pre D3 HO D3(cholecalciferol) tp4Ca 1250H)2D CH2 Liver HO Kidney CH. 24,25(OH)2D3(secalciferol) HO HO 250HD3 CH2 HO人OH 27 1,25(OH)2D3 (calcitriol) Figure 3.Conversion of 7-dehydrocholesterol to vitamin D3 and metabolism of D3 to 1,25(OH)2D3 and 24,25(OH)2D3.Control of the latter step is exerted primarily at the level of the kidney,where low serum phosphorus,low serum calcium,and high parathyroid hormone favor the production of 1,25(OH)2D3.The inset shows the side chain for ergosterol.Ergosterol undergoes similar transformation to vitamin D2 (ergocalciferol),which,in turn,is metabolized to 25(OH)D2,1,25(OH)2D2,and 24,25(OH)2D2.In humans,corresponding D2 and D3 derivatives have equivalent effects and potency.They are therefore referred to in the text without a subscript.0
phosphate transport or bone resorption. However, 25(OH)D appears to be more potent than 1,25(OH)2D in stimulating renal reabsorption of calcium and phosphate and may be the major metabolite regulating calcium flux and contractility in muscle. Specific receptors for 1,25(OH)2D exist in target tissues. However, the role and even the existence of separate receptors for 25(OH)D and 24,25(OH)2D remain controversial. The receptor for 1,25(OH)2D exists in a wide variety of tissues¾not just bone, gut, and kidney. In these "nonclassic" tissues, 1,25(OH)2D exerts a number of actions including regulation of parathyroid hormone secretion from the parathyroid gland, insulin secretion from the pancreas, cytokine production by macrophages and T cells, and proliferation and differentiation of a large number of cells, including cancer cells. Thus, the clinical utility of 1,25(OH)2D analogs is likely to expand. Figure 3. Conversion of 7-dehydrocholesterol to vitamin D3 and metabolism of D3 to 1,25(OH)2D3 and 24,25(OH)2D3. Control of the latter step is exerted primarily at the level of the kidney, where low serum phosphorus, low serum calcium, and high parathyroid hormone favor the production of 1,25(OH)2D3. The inset shows the side chain for ergosterol. Ergosterol undergoes similar transformation to vitamin D2 (ergocalciferol), which, in turn, is metabolized to 25(OH)D2, 1,25(OH)2D2, and 24,25(OH)2D2. In humans, corresponding D2 and D3 derivatives have equivalent effects and potency. They are therefore referred to in the text without a subscript. 0

INTERACTION OF PTH VITAMIN D A summary of the principal actions of PTH and vitamin D on the three main target tissues%intestine,kidney,and bone%is presented in Table 42-2.The net effect of PTH is to raise serum calcium and reduce serum phosphate;the net effect of vitamin D is to raise both.Regulation of calcium and phosphate homeostasis is achieved through a variety of feedback loops.Calcium is the principal regulator of PTH secretion.It binds to a novel ion recognition site that is part of a Gq protein-coupled receptor called the calcium sensing receptor (CaR)and links changes in intracellular free calcium concentration to changes in extracellular calcium.As serum calcium levels rise and bind to this receptor,intracellular calcium levels increase and inhibit PTH secretion.Phosphate regulates PTH secretion directly and indirectly by forming complexes with calcium in the serum.Because it is the ionized free concentration of calcium that is detected by the parathyroid gland,increases in serum phosphate levels reduce the ionized calcium and lead to enhanced PTH secretion.Such feedback regulation is appropriate to the net effect of PTH to raise serum calcium and reduce serum phosphate levels.Likewise,both calcium and phosphate at high levels reduce the amount of 1,25(OH)2D produced by the kidney and increase the amount of 24,25(OH)2D produced.The high calcium works directly and indirectly by reducing PTH secretion.The high phosphate works directly and indirectly by increasing FGF23 levels.Since 1,25(OH)2D raises serum calcium and phosphate,whereas 24,25(OH)2D has less effect,such feedback regulation is again appropriate. 1,25(OH)2D itself directly inhibits PTH secretion (independently of its effect on serum calcium)by a direct action on PTH gene transcription.This provides yet another negative feedback loop.The ability of 1,25(OH)2D to inhibit PTH secretion directly is being exploited using calcitriol analogs that have less effect on serum calcium because of their lesser effect on intestinal calcium absorption.Such drugs are proving useful in the management of secondary hyperparathyroidism accompanying chronic kidney disease and may be useful in selected cases of primary hyperparathyroidism. SECONDARY HORMONAL REGULATORS OF BONE MINERAL HOMEOSTASIS INTRODUCTION A number of hormones modulate the actions of PTH and vitamin D in regulating bone mineral homeostasis.Compared with that of PTH and vitamin D,the physiologic impact of such secondary regulation on bone mineral homeostasis is minor.However, in pharmacologic amounts,a number of these hormones have actions on the bone mineral homeostatic mechanisms that can be exploited therapeutically. CALCITONIN
INTERACTION OF PTH & VITAMIN D A summary of the principal actions of PTH and vitamin D on the three main target tissues¾intestine, kidney, and bone¾is presented in Table 42-2. The net effect of PTH is to raise serum calcium and reduce serum phosphate; the net effect of vitamin D is to raise both. Regulation of calcium and phosphate homeostasis is achieved through a variety of feedback loops. Calcium is the principal regulator of PTH secretion. It binds to a novel ion recognition site that is part of a Gq protein-coupled receptor called the calcium sensing receptor (CaR) and links changes in intracellular free calcium concentration to changes in extracellular calcium. As serum calcium levels rise and bind to this receptor, intracellular calcium levels increase and inhibit PTH secretion. Phosphate regulates PTH secretion directly and indirectly by forming complexes with calcium in the serum. Because it is the ionized free concentration of calcium that is detected by the parathyroid gland, increases in serum phosphate levels reduce the ionized calcium and lead to enhanced PTH secretion. Such feedback regulation is appropriate to the net effect of PTH to raise serum calcium and reduce serum phosphate levels. Likewise, both calcium and phosphate at high levels reduce the amount of 1,25(OH)2D produced by the kidney and increase the amount of 24,25(OH)2D produced. The high calcium works directly and indirectly by reducing PTH secretion. The high phosphate works directly and indirectly by increasing FGF23 levels. Since 1,25(OH)2D raises serum calcium and phosphate, whereas 24,25(OH)2D has less effect, such feedback regulation is again appropriate. 1,25(OH)2D itself directly inhibits PTH secretion (independently of its effect on serum calcium) by a direct action on PTH gene transcription. This provides yet another negative feedback loop. The ability of 1,25(OH)2D to inhibit PTH secretion directly is being exploited using calcitriol analogs that have less effect on serum calcium because of their lesser effect on intestinal calcium absorption. Such drugs are proving useful in the management of secondary hyperparathyroidism accompanying chronic kidney disease and may be useful in selected cases of primary hyperparathyroidism. SECONDARY HORMONAL REGULATORS OF BONE MINERAL HOMEOSTASIS INTRODUCTION A number of hormones modulate the actions of PTH and vitamin D in regulating bone mineral homeostasis. Compared with that of PTH and vitamin D, the physiologic impact of such secondary regulation on bone mineral homeostasis is minor. However, in pharmacologic amounts, a number of these hormones have actions on the bone mineral homeostatic mechanisms that can be exploited therapeutically. CALCITONIN

The calcitonin secreted by the parafollicular cells of the mammalian thyroid is a single-chain peptide hormone with 32 amino acids and a molecular weight of 3600.A disulfide bond between positions 1 and 7 is essential for biologic activity.Calcitonin is produced from a precursor with MW 15,000.The circulating forms of calcitonin are multiple,ranging in size from the monomer (MW 3600)to forms with an apparent molecular weight of 60,000.Whether such heterogeneity includes precursor forms or covalently linked oligomers is not known.Because of its heterogeneity,calcitonin is standardized by bioassay in rats.Activity is compared to a standard maintained by the British Medical Research Council(MRC)and expressed as MRC units. Human calcitonin monomer has a half-life of about 10 minutes with a metabolic clearance of 8-9 mL/kg/min.Salmon calcitonin has a longer half-life and a reduced metabolic clearance(3 mL/kg/min),making it more attractive as a therapeutic agent Much of the clearance occurs in the kidney,although little intact calcitonin appears in the urine The principal effects of calcitonin are to lower serum calcium and phosphate by actions on bone and kidney.Calcitonin inhibits osteoclastic bone resorption.Although bone formation is not impaired at first after calcitonin administration,with time both formation and resorption of bone are reduced.In the kidney,calcitonin reduces both calcium and phosphate reabsorption as well as reabsorption of other ions,including sodium,potassium,and magnesium.Tissues other than bone and kidney are also affected by calcitonin.Calcitonin in pharmacologic amounts decreases gastrin secretion and reduces gastric acid output while increasing secretion of sodium, potassium,chloride,and water in the gut.Pentagastrin is a potent stimulator of calcitonin secretion (as is hypercalcemia),suggesting a possible physiologic relationship between gastrin and calcitonin.In the adult human,no readily demonstrable problem develops in cases of calcitonin deficiency (thyroidectomy)or excess (medullary carcinoma of the thyroid).However,the ability of calcitonin to block bone resorption and lower serum calcium makes it a useful drug for the treatment of Paget's disease,hypercalcemia,and osteoporosis. GLUCOCORTICOIDS Glucocorticoid hormones alter bone mineral homeostasis by antagonizing vitamin D-stimulated intestinal calcium transport,by stimulating renal calcium excretion,and by blocking bone formation.Although these observations underscore the negative impact of glucocorticoids on bone mineral homeostasis,these hormones have proved useful in reversing the hypercalcemia associated with lymphomas and granulomatous diseases such as sarcoidosis (in which production of 1,25[OH]2D is increased)or in cases of vitamin D intoxication.Prolonged administration of glucocorticoids is a common cause of osteoporosis in adults and stunted skeletal development in children. ESTROGENS
The calcitonin secreted by the parafollicular cells of the mammalian thyroid is a single-chain peptide hormone with 32 amino acids and a molecular weight of 3600. A disulfide bond between positions 1 and 7 is essential for biologic activity. Calcitonin is produced from a precursor with MW 15,000. The circulating forms of calcitonin are multiple, ranging in size from the monomer (MW 3600) to forms with an apparent molecular weight of 60,000. Whether such heterogeneity includes precursor forms or covalently linked oligomers is not known. Because of its heterogeneity, calcitonin is standardized by bioassay in rats. Activity is compared to a standard maintained by the British Medical Research Council (MRC) and expressed as MRC units. Human calcitonin monomer has a half-life of about 10 minutes with a metabolic clearance of 8-9 mL/kg/min. Salmon calcitonin has a longer half-life and a reduced metabolic clearance (3 mL/kg/min), making it more attractive as a therapeutic agent. Much of the clearance occurs in the kidney, although little intact calcitonin appears in the urine. The principal effects of calcitonin are to lower serum calcium and phosphate by actions on bone and kidney. Calcitonin inhibits osteoclastic bone resorption. Although bone formation is not impaired at first after calcitonin administration, with time both formation and resorption of bone are reduced. In the kidney, calcitonin reduces both calcium and phosphate reabsorption as well as reabsorption of other ions, including sodium, potassium, and magnesium. Tissues other than bone and kidney are also affected by calcitonin. Calcitonin in pharmacologic amounts decreases gastrin secretion and reduces gastric acid output while increasing secretion of sodium, potassium, chloride, and water in the gut. Pentagastrin is a potent stimulator of calcitonin secretion (as is hypercalcemia), suggesting a possible physiologic relationship between gastrin and calcitonin. In the adult human, no readily demonstrable problem develops in cases of calcitonin deficiency (thyroidectomy) or excess (medullary carcinoma of the thyroid). However, the ability of calcitonin to block bone resorption and lower serum calcium makes it a useful drug for the treatment of Paget's disease, hypercalcemia, and osteoporosis. GLUCOCORTICOIDS Glucocorticoid hormones alter bone mineral homeostasis by antagonizing vitamin D-stimulated intestinal calcium transport, by stimulating renal calcium excretion, and by blocking bone formation. Although these observations underscore the negative impact of glucocorticoids on bone mineral homeostasis, these hormones have proved useful in reversing the hypercalcemia associated with lymphomas and granulomatous diseases such as sarcoidosis (in which production of 1,25[OH]2D is increased) or in cases of vitamin D intoxication. Prolonged administration of glucocorticoids is a common cause of osteoporosis in adults and stunted skeletal development in children. ESTROGENS

Estrogens can prevent accelerated bone loss during the immediate postmenopausal period and at least transiently increase bone in the postmenopausal woman.The prevailing hypothesis advanced to explain these observations is that estrogens reduce the bone-resorbing action of PTH.Estrogen administration leads to an increased 1,25(OH)2D level in blood,but estrogens have no direct effect on 1,25(OH)2D production in vitro.The increased 1,25(OH)2D levels in vivo following estrogen treatment may result from decreased serum calcium and phosphate and increased PTH. Estrogen receptors have been found in bone,and estrogen has direct effects on bone remodeling.Recent case reports of men who lack the estrogen receptor or who are unable to produce estrogen because of aromatase deficiency noted marked osteopenia and failure to close epiphyses.This further substantiates the role of estrogen in bone development,even in men.The principal therapeutic application for estrogen administration in disorders of bone mineral homeostasis is the treatment or prevention of postmenopausal osteoporosis.However,long-term use of estrogen is being discouraged because of its deleterious side effects.Rather,selective estrogen receptor modulators(SERMs)have been developed to retain the beneficial effects on bone while minimizing these deleterious side effects on breast,uterus,and the cardiovascular system (see Box:Newer Therapies for Osteoporosis). NEWER THERAPIES FOR OSTEOPOROSIS Bone undergoes a continuous remodeling process involving bone resorption and formation.Any process that disrupts this balance by increasing resorption relative to formation results in osteoporosis.Inadequate sex hormone production is a major cause of osteoporosis in men and women.Estrogen replacement therapy at menopause is a well-established means of preventing osteoporosis in the female,but many women fear its adverse effects,particularly the increased risk of breast cancer from continued estrogen use (the well-demonstrated increased risk of endometrial cancer is prevented by cycling with a progestin)and do not like the persistence of menstrual bleeding that often accompanies this form of therapy.Medical enthusiasm for this treatment has waned with the demonstration that it does not protect against heart disease. Raloxifene is the first of the selective estrogen receptor modulators (SERMs;see Chapter 40)to be approved for the prevention of osteoporosis.Raloxifene shares some of the beneficial effects of estrogen on bone without increasing the risk of breast or endometrial cancer (it may actually reduce the risk of breast cancer).Although not as effective as estrogen in increasing bone density,raloxifene has been shown to reduce vertebral fractures. Nonhormonal forms of therapy for osteoporosis with proven efficacy in reducing fracture risk have also been developed.Bisphosphonates such as alendronate, risedronate,and ibandronate have been conclusively shown to increase bone density and reduce fractures over at least 5 years when used continuously at a dosage of 10
Estrogens can prevent accelerated bone loss during the immediate postmenopausal period and at least transiently increase bone in the postmenopausal woman. The prevailing hypothesis advanced to explain these observations is that estrogens reduce the bone-resorbing action of PTH. Estrogen administration leads to an increased 1,25(OH)2D level in blood, but estrogens have no direct effect on 1,25(OH)2D production in vitro. The increased 1,25(OH)2D levels in vivo following estrogen treatment may result from decreased serum calcium and phosphate and increased PTH. Estrogen receptors have been found in bone, and estrogen has direct effects on bone remodeling. Recent case reports of men who lack the estrogen receptor or who are unable to produce estrogen because of aromatase deficiency noted marked osteopenia and failure to close epiphyses. This further substantiates the role of estrogen in bone development, even in men. The principal therapeutic application for estrogen administration in disorders of bone mineral homeostasis is the treatment or prevention of postmenopausal osteoporosis. However, long-term use of estrogen is being discouraged because of its deleterious side effects. Rather, selective estrogen receptor modulators (SERMs) have been developed to retain the beneficial effects on bone while minimizing these deleterious side effects on breast, uterus, and the cardiovascular system (see Box: Newer Therapies for Osteoporosis). NEWER THERAPIES FOR OSTEOPOROSIS Bone undergoes a continuous remodeling process involving bone resorption and formation. Any process that disrupts this balance by increasing resorption relative to formation results in osteoporosis. Inadequate sex hormone production is a major cause of osteoporosis in men and women. Estrogen replacement therapy at menopause is a well-established means of preventing osteoporosis in the female, but many women fear its adverse effects, particularly the increased risk of breast cancer from continued estrogen use (the well-demonstrated increased risk of endometrial cancer is prevented by cycling with a progestin) and do not like the persistence of menstrual bleeding that often accompanies this form of therapy. Medical enthusiasm for this treatment has waned with the demonstration that it does not protect against heart disease. Raloxifene is the first of the selective estrogen receptor modulators (SERMs; see Chapter 40) to be approved for the prevention of osteoporosis. Raloxifene shares some of the beneficial effects of estrogen on bone without increasing the risk of breast or endometrial cancer (it may actually reduce the risk of breast cancer). Although not as effective as estrogen in increasing bone density, raloxifene has been shown to reduce vertebral fractures. Nonhormonal forms of therapy for osteoporosis with proven efficacy in reducing fracture risk have also been developed. Bisphosphonates such as alendronate, risedronate, and ibandronate have been conclusively shown to increase bone density and reduce fractures over at least 5 years when used continuously at a dosage of 10

mg/d or 70 mg/wk for alendronate and 5 mg/d or 35 mg/wk for risedronate,2.5 mg/d or 150 mg/mo for ibandronate.Side-by-side trials between alendronate and calcitonin (another approved nonestrogen drug for osteoporosis)indicated a greater efficacy of alendronate.Bisphosphonates are poorly absorbed and must be given on an empty stomach or infused intravenously.At the higher oral doses used in the treatment of Paget's disease,alendronate causes gastric irritation,but this is not a significant problem at the doses recommended for osteoporosis when patients are instructed to take the drug with a glass of water and remain upright.The most recently approved drug for osteoporosis is teriparatide,the recombinant form of PTH1-34.Unlike other approved drugs for osteoporosis,teriparatide stimulates bone formation rather than inhibiting bone resorption.However,teriparatide must be given daily by subcutaneous injection.Its efficacy in preventing fractures appears to be at least as great as that of the bisphosphonates.In all cases,adequate intake of calcium and vitamin D needs to be maintained. Thus,we now have several well-validated,efficacious forms of treatment for this common debilitating disease. NONHORMONALAGENTS AFFECTING BONE MINERAL HOMEOSTASIS BISPHOSPHONATES The bisphosphonates are analogs of pyrophosphate in which the P-O-P bond has been replaced with a nonhydrolyzable P-C-P bond(Figure 4).Etidronate,pamidronate,and alendronate have now been joined by risedronate,tiludronate,ibandronate,and zoledronate for clinical use.The bisphosphonates owe at least part of their clinical usefulness and toxicity to their ability to retard formation and dissolution of hydroxyapatite crystals within and outside the skeletal system.They localize to regions of bone resorption and so exert their greatest effects on osteoclasts.However, the exact mechanism by which they selectively inhibit bone resorption is not clear. The results from animal and clinical studies indicate that less than 10%of an oral dose of these drugs is absorbed.Food reduces absorption even further,necessitating their administration on an empty stomach.Because it causes gastric irritation, pamidronate is not available as an oral preparation.However,with the possible exception of etidronate,all currently available bisphosphonates have this complication. Nearly half of the absorbed drug accumulates in bone;the remainder is excreted unchanged in the urine.Decreased renal function,esophageal motility disorders,and peptic ulcer disease are the main contraindications to the use of these drugs.The portion bound to bone is retained for months,depending on the turnover of bone itself. Etidronate and the other bisphosphonates exert a variety of effects on bone mineral homeostasis.In particular,bisphosphonates are useful for the treatment of
mg/d or 70 mg/wk for alendronate and 5 mg/d or 35 mg/wk for risedronate, 2.5 mg/d or 150 mg/mo for ibandronate. Side-by-side trials between alendronate and calcitonin (another approved nonestrogen drug for osteoporosis) indicated a greater efficacy of alendronate. Bisphosphonates are poorly absorbed and must be given on an empty stomach or infused intravenously. At the higher oral doses used in the treatment of Paget's disease, alendronate causes gastric irritation, but this is not a significant problem at the doses recommended for osteoporosis when patients are instructed to take the drug with a glass of water and remain upright. The most recently approved drug for osteoporosis is teriparatide, the recombinant form of PTH1-34. Unlike other approved drugs for osteoporosis, teriparatide stimulates bone formation rather than inhibiting bone resorption. However, teriparatide must be given daily by subcutaneous injection. Its efficacy in preventing fractures appears to be at least as great as that of the bisphosphonates. In all cases, adequate intake of calcium and vitamin D needs to be maintained. Thus, we now have several well-validated, efficacious forms of treatment for this common debilitating disease. NONHORMONAL AGENTS AFFECTING BONE MINERAL HOMEOSTASIS BISPHOSPHONATES The bisphosphonates are analogs of pyrophosphate in which the P-O-P bond has been replaced with a nonhydrolyzable P-C-P bond (Figure 4). Etidronate, pamidronate, and alendronate have now been joined by risedronate, tiludronate, ibandronate, and zoledronate for clinical use. The bisphosphonates owe at least part of their clinical usefulness and toxicity to their ability to retard formation and dissolution of hydroxyapatite crystals within and outside the skeletal system. They localize to regions of bone resorption and so exert their greatest effects on osteoclasts. However, the exact mechanism by which they selectively inhibit bone resorption is not clear. The results from animal and clinical studies indicate that less than 10% of an oral dose of these drugs is absorbed. Food reduces absorption even further, necessitating their administration on an empty stomach. Because it causes gastric irritation, pamidronate is not available as an oral preparation. However, with the possible exception of etidronate, all currently available bisphosphonates have this complication. Nearly half of the absorbed drug accumulates in bone; the remainder is excreted unchanged in the urine. Decreased renal function, esophageal motility disorders, and peptic ulcer disease are the main contraindications to the use of these drugs. The portion bound to bone is retained for months, depending on the turnover of bone itself. Etidronate and the other bisphosphonates exert a variety of effects on bone mineral homeostasis. In particular, bisphosphonates are useful for the treatment of