
English Reading Materials Chapter 26:Drugs Used in Asthma INTRODUCTION Asthma is characterized clinically by recurrent bouts of coughing,shortness of breath, chest tightness,and wheezing;physiologically by widespread,reversible narrowing of the bronchial airways and a marked increase in bronchial responsiveness to inhaled stimuli;and pathologically by lymphocytic,eosinophilic inflammation of the bronchial mucosa.It is also characterized pathologically by remodeling of the bronchial mucosa,with deposition of collagen beneath the epithelium's lamina reticularis and hyperplasia of the cells of all structural elementsvessels,smooth muscle,and secretory glands and goblet cells. In mild asthma,symptoms occur only occasionally,as on exposure to allergens or certain pollutants,on exercise,or after a viral upper respiratory infection.More severe forms of asthma are associated with frequent attacks of wheezing dyspnea,especially at night,and may be associated with chronic airway narrowing,causing chronic respiratory impairment.These consequences of asthma are regarded as largely preventable,because effective treatments for relief of acute bronchoconstriction ("short term relievers")and for reduction in symptoms and prevention of attacks ("long-term controllers")are available (but underutilized). The causes of airway narrowing in acute asthmatic attacks include contraction of airway smooth muscle,inspissation of thick,viscid mucus plugs in the airway lumen, and thickening of the bronchial mucosa from edema,cellular infiltration,and hyperplasia of secretory,vascular,and smooth muscle cells.Of these causes of airway obstruction,contraction of smooth muscle is most easily reversed by current therapy; reversal of the edema and cellular infiltration requires sustained treatment with anti-inflammatory agents. Short-term relief is thus most effectively achieved by agents that relax airway smooth muscle,of which -adrenoceptor stimulants are the most effective and most widely used.Theophylline,a methylxanthine drug,and antimuscarinic agents are also used for reversal of airway constriction Long-term control is most effectively achieved with an anti-inflammatory agent such as an inhaled corticosteroid.It can also be achieved,though less effectively,with a leukotriene pathway antagonist or an inhibitor of mast cell degranulation,such as cromolyn or nedocromil.Finally,clinical trials have established the efficacy of treatment for asthma with a humanized monoclonal antibody,omalizumab,which is specifically targeted against IgE,the antibody responsible for allergic sensitization
English Reading Materials Chapter 26: Drugs Used in Asthma INTRODUCTION Asthma is characterized clinically by recurrent bouts of coughing, shortness of breath, chest tightness, and wheezing; physiologically by widespread, reversible narrowing of the bronchial airways and a marked increase in bronchial responsiveness to inhaled stimuli; and pathologically by lymphocytic, eosinophilic inflammation of the bronchial mucosa. It is also characterized pathologically by remodeling of the bronchial mucosa, with deposition of collagen beneath the epithelium's lamina reticularis and hyperplasia of the cells of all structural elementsvessels, smooth muscle, and secretory glands and goblet cells. In mild asthma, symptoms occur only occasionally, as on exposure to allergens or certain pollutants, on exercise, or after a viral upper respiratory infection. More severe forms of asthma are associated with frequent attacks of wheezing dyspnea, especially at night, and may be associated with chronic airway narrowing, causing chronic respiratory impairment. These consequences of asthma are regarded as largely preventable, because effective treatments for relief of acute bronchoconstriction ("short term relievers") and for reduction in symptoms and prevention of attacks ("long-term controllers") are available (but underutilized). The causes of airway narrowing in acute asthmatic attacks include contraction of airway smooth muscle, inspissation of thick, viscid mucus plugs in the airway lumen, and thickening of the bronchial mucosa from edema, cellular infiltration, and hyperplasia of secretory, vascular, and smooth muscle cells. Of these causes of airway obstruction, contraction of smooth muscle is most easily reversed by current therapy; reversal of the edema and cellular infiltration requires sustained treatment with anti-inflammatory agents. Short-term relief is thus most effectively achieved by agents that relax airway smooth muscle, of which -adrenoceptor stimulants are the most effective and most widely used. Theophylline, a methylxanthine drug, and antimuscarinic agents are also used for reversal of airway constriction. Long-term control is most effectively achieved with an anti-inflammatory agent such as an inhaled corticosteroid. It can also be achieved, though less effectively, with a leukotriene pathway antagonist or an inhibitor of mast cell degranulation, such as cromolyn or nedocromil. Finally, clinical trials have established the efficacy of treatment for asthma with a humanized monoclonal antibody, omalizumab, which is specifically targeted against IgE, the antibody responsible for allergic sensitization

The distinction between "short-term relievers"and "long-term controllers"has become blurred.Theophylline,regarded as a bronchodilator,inhibits some lymphocyte functions and modestly reduces airway mucosal inflammation.Inhaled corticosteroids,regarded as long-term controllers,produce modest immediate bronchodilation.Two recently released long-acting -adrenoceptor stimulants, salmeterol and formoterol,appear to be effective in improving asthma control when added to inhaled corticosteroid treatment. This chapter presents the basic pharmacology of the methylxanthines,cromolyn, leukotriene pathway inhibitors,and monoclonal anti-IgE antibody -agents whose medical use is almost exclusively for pulmonary disease.The other classes of drugs listed above are discussed in relation to the therapy of asthma. PATHOGENESIS OF ASTHMA The classic immunologic model of asthma presents it as a disease mediated by reaginic immune globulin (IgE).Foreign materials that provoke IgE production are described as "allergens";the most common are proteins from house dust mite, cockroach,cat dander,molds,and pollens.The tendency to produce IgE antibodies is genetically determined;asthma and other allergic diseases cluster in families.Once produced,IgE antibodies bind to mast cells in the airway mucosa (Figure 1).On reexposure to a specific allergen,antigen-antibody interaction on the surface of the mast cells triggers both the release of mediators stored in the cells'granules and the synthesis and release of other mediators.The histamine,tryptase,leukotrienes C4 and D4,and prostaglandin D2,when released,diffuse through the airway mucosa triggering the muscle contraction and vascular leakage responsible for the acute bronchoconstriction of the "early asthmatic response."This response is often followed in 4-6 hours by a second,more sustained phase of bronchoconstriction,the "late asthmatic response,"which is associated with an influx of inflammatory cells into the bronchial mucosa and with an increase in bronchial responsiveness that may last for several weeks after a single inhalation of allergen.The mediators responsible for this late response are thought to be cytokines characteristically produced by TH2 lymphocytes,especially interleukins 5,9,and 13.The cytokines are thought to attract and activate eosinophils,stimulate IgE production by B lymphocytes,and directly stimulate mucus production by bronchial epithelial cells.It is not clear whether lymphocytes or mast cells in the airway mucosa are the primary source of the mediators responsible for the late inflammatory response,but the benefits of corticosteroid therapy are attributed to their inhibition of cytokine production in the airways The allergen challenge model does not reproduce all the features of asthma.Most asthma attacks are not triggered by inhalation of allergens.They are triggered by viral respiratory infection.Some adults with asthma have no evidence of allergic sensitivity
The distinction between "short-term relievers" and "long-term controllers" has become blurred. Theophylline, regarded as a bronchodilator, inhibits some lymphocyte functions and modestly reduces airway mucosal inflammation. Inhaled corticosteroids, regarded as long-term controllers, produce modest immediate bronchodilation. Two recently released long-acting -adrenoceptor stimulants, salmeterol and formoterol, appear to be effective in improving asthma control when added to inhaled corticosteroid treatment. This chapter presents the basic pharmacology of the methylxanthines, cromolyn, leukotriene pathway inhibitors, and monoclonal anti-IgE antibody - agents whose medical use is almost exclusively for pulmonary disease. The other classes of drugs listed above are discussed in relation to the therapy of asthma. PATHOGENESIS OF ASTHMA The classic immunologic model of asthma presents it as a disease mediated by reaginic immune globulin (IgE). Foreign materials that provoke IgE production are described as "allergens"; the most common are proteins from house dust mite, cockroach, cat dander, molds, and pollens. The tendency to produce IgE antibodies is genetically determined; asthma and other allergic diseases cluster in families. Once produced, IgE antibodies bind to mast cells in the airway mucosa (Figure 1). On reexposure to a specific allergen, antigen-antibody interaction on the surface of the mast cells triggers both the release of mediators stored in the cells' granules and the synthesis and release of other mediators. The histamine, tryptase, leukotrienes C4 and D4, and prostaglandin D2, when released, diffuse through the airway mucosa triggering the muscle contraction and vascular leakage responsible for the acute bronchoconstriction of the "early asthmatic response." This response is often followed in 4-6 hours by a second, more sustained phase of bronchoconstriction, the "late asthmatic response," which is associated with an influx of inflammatory cells into the bronchial mucosa and with an increase in bronchial responsiveness that may last for several weeks after a single inhalation of allergen. The mediators responsible for this late response are thought to be cytokines characteristically produced by TH2 lymphocytes, especially interleukins 5, 9, and 13. The cytokines are thought to attract and activate eosinophils, stimulate IgE production by B lymphocytes, and directly stimulate mucus production by bronchial epithelial cells. It is not clear whether lymphocytes or mast cells in the airway mucosa are the primary source of the mediators responsible for the late inflammatory response, but the benefits of corticosteroid therapy are attributed to their inhibition of cytokine production in the airways. The allergen challenge model does not reproduce all the features of asthma. Most asthma attacks are not triggered by inhalation of allergens. They are triggered by viral respiratory infection. Some adults with asthma have no evidence of allergic sensitivity

to allergens,and even in people with allergic sensitivity,the severity of symptoms correlates poorly with levels of allergen in the atmosphere.Moreover,bronchospasm can be provoked by nonallergenic stimuli such as distilled water,exercise,cold air, sulfur dioxide,and rapid respiratory maneuvers. This tendency to develop bronchospasm on encountering stimuli that do not affect healthy nonasthmatic airways is characteristic of asthma and is sometimes called "nonspecific bronchial hyperreactivity"to distinguish it from bronchial responsiveness to specific antigens.Bronchial reactivity is assessed by measuring the fall in forced expiratory volume in 1 second(FEVI)provoked by inhaling serially increasing concentrations of aerosolized methacholine.The exaggerated reactivity of the airways appears to be fundamental to asthma's pathogenesis,because it is nearly ubiquitous in patients with asthma and its degree correlates with the clinical severity of the disease. The mechanisms underlying bronchial hyperreactivity are somehow related to inflammation of the airway mucosa.The agents that increase bronchial reactivity, such as ozone exposure,allergen inhalation,and infection with respiratory viruses, also cause airway inflammation.The increase in reactivity due to allergen inhalation is associated with an increase in both eosinophils and polymorphonuclear leukocytes in bronchial lavage fluid.The increase in reactivity that is associated with the late asthmatic response to allergen inhalation (Figure 1)is sustained and,because it is prevented by treatment with an inhaled corticosteroid,is thought to be caused by airway inflammation. Whatever the mechanisms responsible for bronchial hyperreactivity, bronchoconstriction itself seems to result not simply from the direct effect of the released mediators but also from their activation of neural or humoral pathways. Evidence for the importance of neural pathways stems largely from studies of laboratory animals.The bronchospasm provoked in dogs by inhalation of histamine is reduced by pretreatment with an inhaled topical anesthetic agent,by transection of the vagus nerves,and by pretreatment with atropine.Studies of asthmatic humans, however,have shown that treatment with atropine causes only a reduction in not abolition ofthe bronchospastic responses to antigens and to nonantigenic stimuli.It is possible that activity in another neural pathway,such as the nonadrenergic, noncholinergic system,contributes to bronchomotor responses stimuli(Figure 2). The hypothesis suggested by these studies that asthmatic bronchospasm results from a combination of release of mediators and an exaggeration of responsiveness to their effects predicts that asthma may be effectively treated by drugs with different modes of action.Asthmatic bronchospasm might be reversed or prevented,for example,by drugs that reduce the amount of IgE bound to mast cells (anti-IgE antibody),prevent mast cell degranulation (cromolyn or nedocromil, sympathomimetic agents,calcium channel blockers),block the action of the products
to allergens, and even in people with allergic sensitivity, the severity of symptoms correlates poorly with levels of allergen in the atmosphere. Moreover, bronchospasm can be provoked by nonallergenic stimuli such as distilled water, exercise, cold air, sulfur dioxide, and rapid respiratory maneuvers. This tendency to develop bronchospasm on encountering stimuli that do not affect healthy nonasthmatic airways is characteristic of asthma and is sometimes called "nonspecific bronchial hyperreactivity" to distinguish it from bronchial responsiveness to specific antigens. Bronchial reactivity is assessed by measuring the fall in forced expiratory volume in 1 second (FEV1) provoked by inhaling serially increasing concentrations of aerosolized methacholine. The exaggerated reactivity of the airways appears to be fundamental to asthma's pathogenesis, because it is nearly ubiquitous in patients with asthma and its degree correlates with the clinical severity of the disease. The mechanisms underlying bronchial hyperreactivity are somehow related to inflammation of the airway mucosa. The agents that increase bronchial reactivity, such as ozone exposure, allergen inhalation, and infection with respiratory viruses, also cause airway inflammation. The increase in reactivity due to allergen inhalation is associated with an increase in both eosinophils and polymorphonuclear leukocytes in bronchial lavage fluid. The increase in reactivity that is associated with the late asthmatic response to allergen inhalation (Figure 1) is sustained and, because it is prevented by treatment with an inhaled corticosteroid, is thought to be caused by airway inflammation. Whatever the mechanisms responsible for bronchial hyperreactivity, bronchoconstriction itself seems to result not simply from the direct effect of the released mediators but also from their activation of neural or humoral pathways. Evidence for the importance of neural pathways stems largely from studies of laboratory animals. The bronchospasm provoked in dogs by inhalation of histamine is reduced by pretreatment with an inhaled topical anesthetic agent, by transection of the vagus nerves, and by pretreatment with atropine. Studies of asthmatic humans, however, have shown that treatment with atropine causes only a reduction in not abolition ofthe bronchospastic responses to antigens and to nonantigenic stimuli. It is possible that activity in another neural pathway, such as the nonadrenergic, noncholinergic system, contributes to bronchomotor responses stimuli (Figure 2). The hypothesis suggested by these studies that asthmatic bronchospasm results from a combination of release of mediators and an exaggeration of responsiveness to their effects predicts that asthma may be effectively treated by drugs with different modes of action. Asthmatic bronchospasm might be reversed or prevented, for example, by drugs that reduce the amount of IgE bound to mast cells (anti-IgE antibody), prevent mast cell degranulation (cromolyn or nedocromil, sympathomimetic agents, calcium channel blockers), block the action of the products
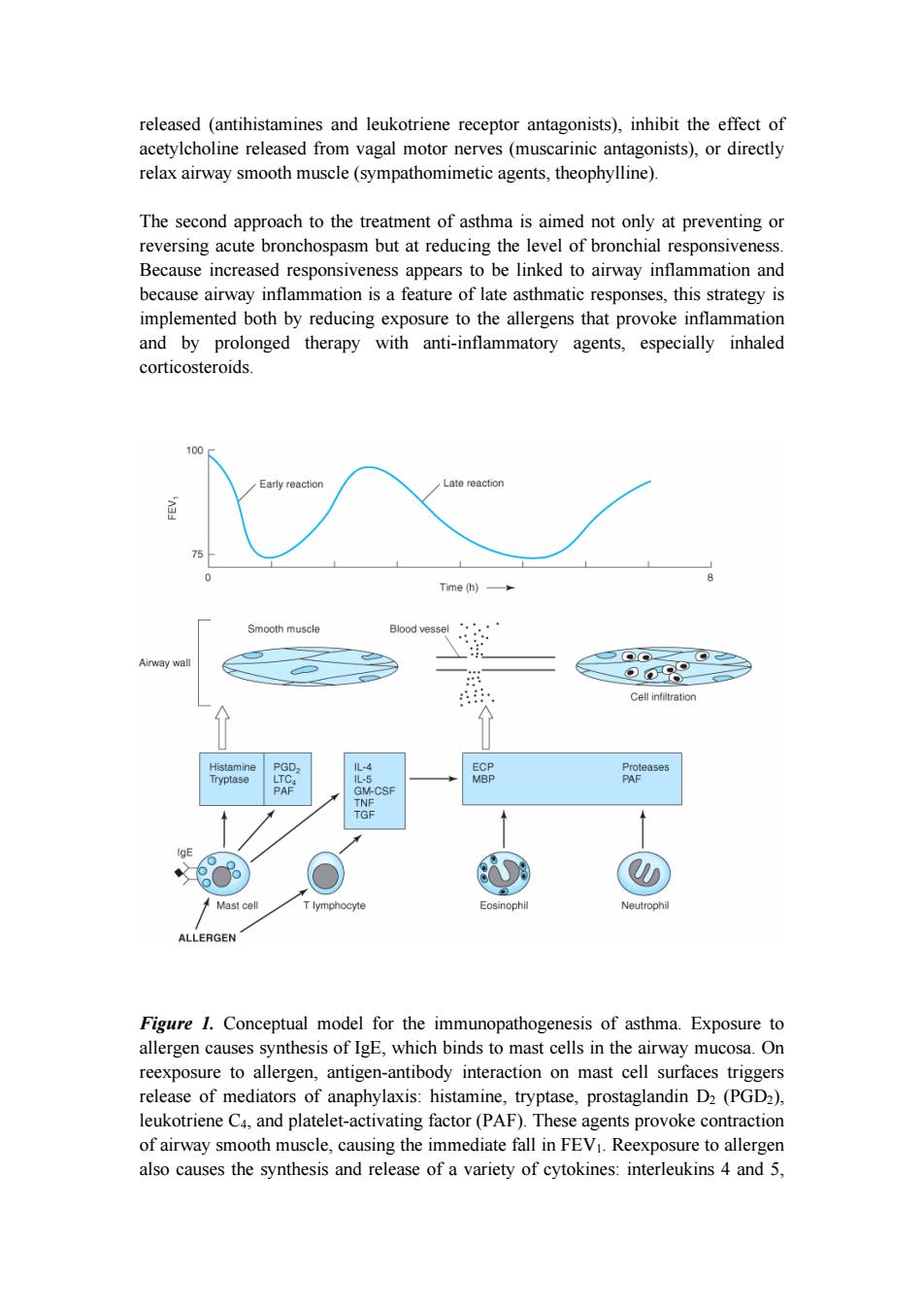
released (antihistamines and leukotriene receptor antagonists),inhibit the effect of acetylcholine released from vagal motor nerves (muscarinic antagonists),or directly relax airway smooth muscle (sympathomimetic agents,theophylline). The second approach to the treatment of asthma is aimed not only at preventing or reversing acute bronchospasm but at reducing the level of bronchial responsiveness. Because increased responsiveness appears to be linked to airway inflammation and because airway inflammation is a feature of late asthmatic responses,this strategy is implemented both by reducing exposure to the allergens that provoke inflammation and by prolonged therapy with anti-inflammatory agents,especially inhaled corticosteroids. 100 Early reaction Late reaction 75 Time (h)多 Smooth muscle Blood vessel Airway wal ⊙09 Cell infiltration Histamine PGD, 儿-4 ECP Proteases Tryptase LTC. L-5 MBP PAF PAF GM-CSF TNF TGF Mast cell T lymphocyte Eosinophil Neutrophil ALLERGEN Figure 1.Conceptual model for the immunopathogenesis of asthma.Exposure to allergen causes synthesis of IgE,which binds to mast cells in the airway mucosa.On reexposure to allergen,antigen-antibody interaction on mast cell surfaces triggers release of mediators of anaphylaxis:histamine,tryptase,prostaglandin D2(PGD2), leukotriene C4,and platelet-activating factor(PAF).These agents provoke contraction of airway smooth muscle,causing the immediate fall in FEV1.Reexposure to allergen also causes the synthesis and release of a variety of cytokines:interleukins 4 and 5
released (antihistamines and leukotriene receptor antagonists), inhibit the effect of acetylcholine released from vagal motor nerves (muscarinic antagonists), or directly relax airway smooth muscle (sympathomimetic agents, theophylline). The second approach to the treatment of asthma is aimed not only at preventing or reversing acute bronchospasm but at reducing the level of bronchial responsiveness. Because increased responsiveness appears to be linked to airway inflammation and because airway inflammation is a feature of late asthmatic responses, this strategy is implemented both by reducing exposure to the allergens that provoke inflammation and by prolonged therapy with anti-inflammatory agents, especially inhaled corticosteroids. Figure 1. Conceptual model for the immunopathogenesis of asthma. Exposure to allergen causes synthesis of IgE, which binds to mast cells in the airway mucosa. On reexposure to allergen, antigen-antibody interaction on mast cell surfaces triggers release of mediators of anaphylaxis: histamine, tryptase, prostaglandin D2 (PGD2), leukotriene C4, and platelet-activating factor (PAF). These agents provoke contraction of airway smooth muscle, causing the immediate fall in FEV1. Reexposure to allergen also causes the synthesis and release of a variety of cytokines: interleukins 4 and 5
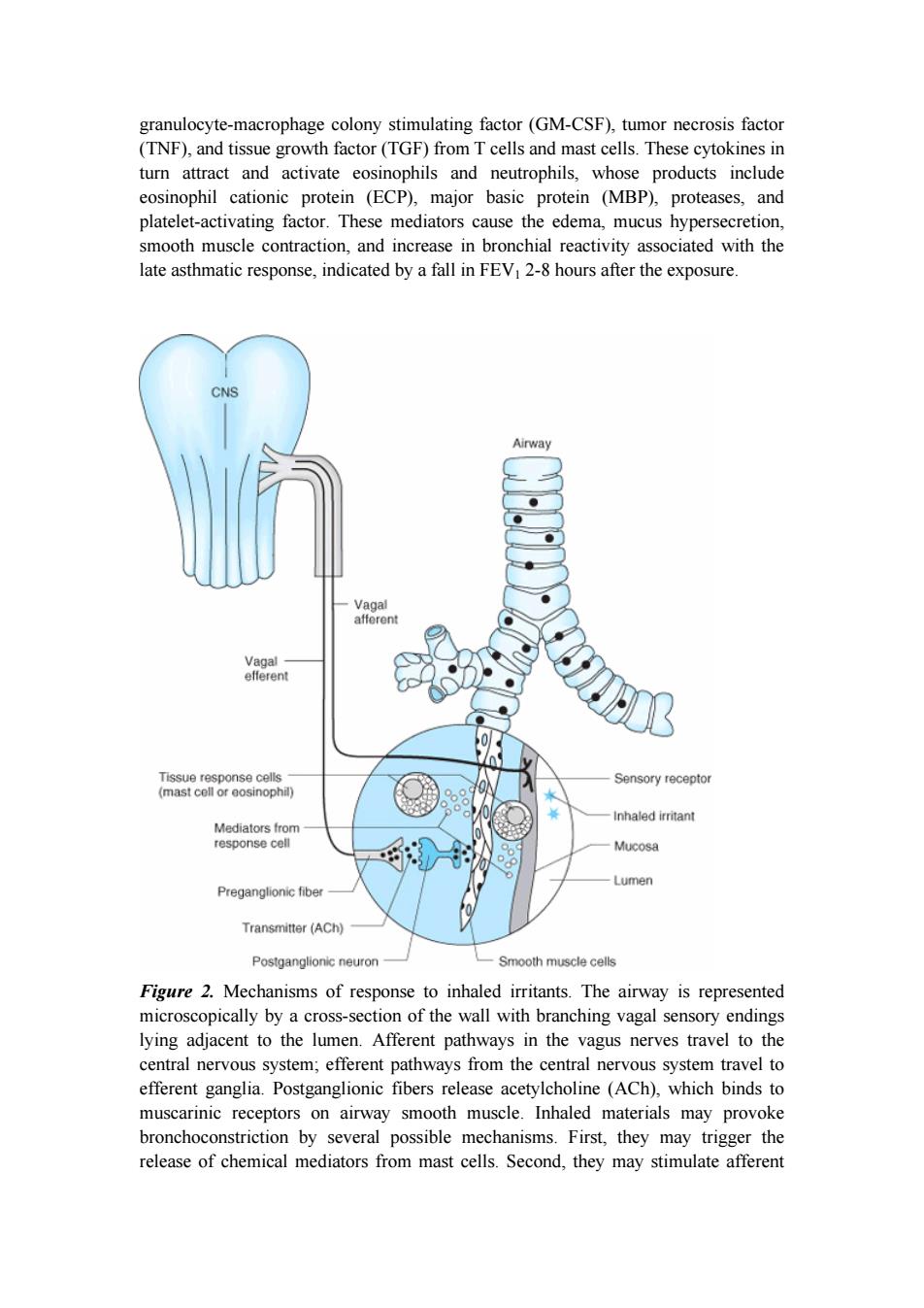
granulocyte-macrophage colony stimulating factor(GM-CSF),tumor necrosis factor (TNF),and tissue growth factor(TGF)from T cells and mast cells.These cytokines in turn attract and activate eosinophils and neutrophils,whose products include eosinophil cationic protein (ECP),major basic protein (MBP),proteases,and platelet-activating factor.These mediators cause the edema,mucus hypersecretion, smooth muscle contraction,and increase in bronchial reactivity associated with the late asthmatic response,indicated by a fall in FEV1 2-8 hours after the exposure. CNS Airway Vagal afferent Vagal efferent Tissue response cells Sensory receptor (mast cell or eosinophil) Inhaled irritant Mediators from response cell Mucosa Lumen Preganglionic fiber 0 Transmitter (ACh) Postganglionic neuron Smooth muscle cells Figure 2.Mechanisms of response to inhaled irritants.The airway is represented microscopically by a cross-section of the wall with branching vagal sensory endings lying adjacent to the lumen.Afferent pathways in the vagus nerves travel to the central nervous system;efferent pathways from the central nervous system travel to efferent ganglia.Postganglionic fibers release acetylcholine (ACh),which binds to muscarinic receptors on airway smooth muscle.Inhaled materials may provoke bronchoconstriction by several possible mechanisms.First,they may trigger the release of chemical mediators from mast cells.Second,they may stimulate afferent
granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor (TNF), and tissue growth factor (TGF) from T cells and mast cells. These cytokines in turn attract and activate eosinophils and neutrophils, whose products include eosinophil cationic protein (ECP), major basic protein (MBP), proteases, and platelet-activating factor. These mediators cause the edema, mucus hypersecretion, smooth muscle contraction, and increase in bronchial reactivity associated with the late asthmatic response, indicated by a fall in FEV1 2-8 hours after the exposure. Figure 2. Mechanisms of response to inhaled irritants. The airway is represented microscopically by a cross-section of the wall with branching vagal sensory endings lying adjacent to the lumen. Afferent pathways in the vagus nerves travel to the central nervous system; efferent pathways from the central nervous system travel to efferent ganglia. Postganglionic fibers release acetylcholine (ACh), which binds to muscarinic receptors on airway smooth muscle. Inhaled materials may provoke bronchoconstriction by several possible mechanisms. First, they may trigger the release of chemical mediators from mast cells. Second, they may stimulate afferent

receptors to initiate reflex bronchoconstriction or to release tachykinins(eg,substance P)that directly stimulate smooth muscle contraction. I.BASIC PHARMACOLOGY OF AGENTS USED IN THE TREATMENT OF ASTHMA INTRODUCTION The drugs most used for management of asthma are adrenoceptor agonists,or sympathomimetic agents (used as "relievers"or bronchodilators)and inhaled corticosteroids (used as "controllers"or anti-inflammatory agents).Their basic pharmacology is presented elsewhere.In this chapter,we review their pharmacology relevant to asthma. SYMPATHOMIMETIC AGENTS Introduction The adrenoceptor agonists have several pharmacologic actions that are important in the treatment of asthma.They relax airway smooth muscle and inhibit release of bronchoconstricting mediators from mast cells.They may also inhibit microvascular leakage and increase mucociliary transport by increasing ciliary activity.As in other tissues,the agonists stimulate adenylyl cyclase and increase the formation of intracellular cAMP(Figure 3) The best-characterized action of the adrenoceptor agonists in the airways is relaxation of airway smooth muscle.Although there is no evidence for significant sympathetic innervation of human airway smooth muscle,ample evidence exists for the presence of adrenoceptors on airway smooth muscle.In general,stimulation of 2 receptors relaxes airway smooth muscle,inhibits mediator release,and causes tachycardia and skeletal muscle tremor as side effects. The sympathomimetic agents that have been widely used in the treatment of asthma include epinephrine,ephedrine,isoproterenol,and albuterol and other 2-selective agents(Figure 4).Because epinephrine and isoproterenol increase the rate and force of cardiac contraction (mediated mainly by receptors),they are reserved for special situations(see below). In general,adrenoceptor agonists are best delivered by inhalation because this results in the greatest local effect on airway smooth muscle with the least systemic toxicity. Aerosol deposition depends on the particle size,the pattern of breathing(tidal volume and rate of airflow),and the geometry of the airways.Even with particles in the optimal size range of 2-5 mm,80-90%of the total dose of aerosol is deposited in the
receptors to initiate reflex bronchoconstriction or to release tachykinins (eg, substance P) that directly stimulate smooth muscle contraction. I. BASIC PHARMACOLOGY OF AGENTS USED IN THE TREATMENT OF ASTHMA INTRODUCTION The drugs most used for management of asthma are adrenoceptor agonists, or sympathomimetic agents (used as "relievers" or bronchodilators) and inhaled corticosteroids (used as "controllers" or anti-inflammatory agents). Their basic pharmacology is presented elsewhere. In this chapter, we review their pharmacology relevant to asthma. SYMPATHOMIMETIC AGENTS Introduction The adrenoceptor agonists have several pharmacologic actions that are important in the treatment of asthma. They relax airway smooth muscle and inhibit release of bronchoconstricting mediators from mast cells. They may also inhibit microvascular leakage and increase mucociliary transport by increasing ciliary activity. As in other tissues, the agonists stimulate adenylyl cyclase and increase the formation of intracellular cAMP (Figure 3). The best-characterized action of the adrenoceptor agonists in the airways is relaxation of airway smooth muscle. Although there is no evidence for significant sympathetic innervation of human airway smooth muscle, ample evidence exists for the presence of adrenoceptors on airway smooth muscle. In general, stimulation of 2 receptors relaxes airway smooth muscle, inhibits mediator release, and causes tachycardia and skeletal muscle tremor as side effects. The sympathomimetic agents that have been widely used in the treatment of asthma include epinephrine, ephedrine, isoproterenol, and albuterol and other 2-selective agents (Figure 4). Because epinephrine and isoproterenol increase the rate and force of cardiac contraction (mediated mainly by 1 receptors), they are reserved for special situations (see below). In general, adrenoceptor agonists are best delivered by inhalation because this results in the greatest local effect on airway smooth muscle with the least systemic toxicity. Aerosol deposition depends on the particle size, the pattern of breathing (tidal volume and rate of airflow), and the geometry of the airways. Even with particles in the optimal size range of 2-5 mm, 80-90% of the total dose of aerosol is deposited in the
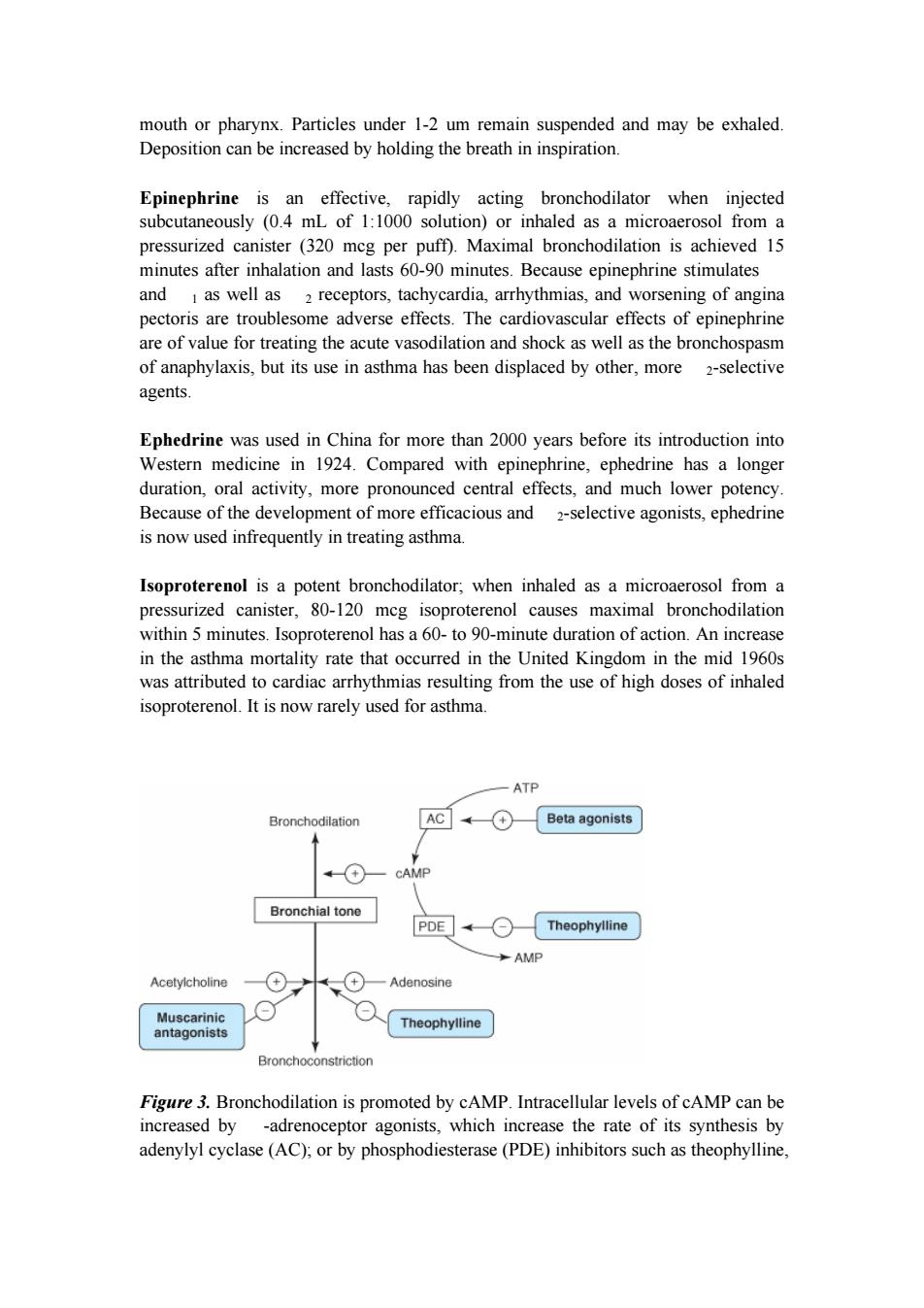
mouth or pharynx.Particles under 1-2 um remain suspended and may be exhaled. Deposition can be increased by holding the breath in inspiration. Epinephrine is an effective,rapidly acting bronchodilator when injected subcutaneously (0.4 mL of 1:1000 solution)or inhaled as a microaerosol from a pressurized canister (320 mcg per puff).Maximal bronchodilation is achieved 15 minutes after inhalation and lasts 60-90 minutes.Because epinephrine stimulates and I as well as 2 receptors,tachycardia,arrhythmias,and worsening of angina pectoris are troublesome adverse effects.The cardiovascular effects of epinephrine are of value for treating the acute vasodilation and shock as well as the bronchospasm of anaphylaxis,but its use in asthma has been displaced by other,more 2-selective agents. Ephedrine was used in China for more than 2000 years before its introduction into Western medicine in 1924.Compared with epinephrine,ephedrine has a longer duration,oral activity,more pronounced central effects,and much lower potency. Because of the development of more efficacious and 2-selective agonists,ephedrine is now used infrequently in treating asthma. Isoproterenol is a potent bronchodilator;when inhaled as a microaerosol from a pressurized canister,80-120 mcg isoproterenol causes maximal bronchodilation within 5 minutes.Isoproterenol has a 60-to 90-minute duration of action.An increase in the asthma mortality rate that occurred in the United Kingdom in the mid 1960s was attributed to cardiac arrhythmias resulting from the use of high doses of inhaled isoproterenol.It is now rarely used for asthma. ATP Bronchodilation AC (+ Beta agonists A + CAMP Bronchial tone PDE Theophylline AMP Acetylcholine Adenosine Muscarinic Theophylline antagonists Bronchoconstriction Figure 3.Bronchodilation is promoted by cAMP.Intracellular levels of cAMP can be increased by -adrenoceptor agonists,which increase the rate of its synthesis by adenylyl cyclase(AC);or by phosphodiesterase(PDE)inhibitors such as theophylline
mouth or pharynx. Particles under 1-2 um remain suspended and may be exhaled. Deposition can be increased by holding the breath in inspiration. Epinephrine is an effective, rapidly acting bronchodilator when injected subcutaneously (0.4 mL of 1:1000 solution) or inhaled as a microaerosol from a pressurized canister (320 mcg per puff). Maximal bronchodilation is achieved 15 minutes after inhalation and lasts 60-90 minutes. Because epinephrine stimulates and 1 as well as 2 receptors, tachycardia, arrhythmias, and worsening of angina pectoris are troublesome adverse effects. The cardiovascular effects of epinephrine are of value for treating the acute vasodilation and shock as well as the bronchospasm of anaphylaxis, but its use in asthma has been displaced by other, more 2-selective agents. Ephedrine was used in China for more than 2000 years before its introduction into Western medicine in 1924. Compared with epinephrine, ephedrine has a longer duration, oral activity, more pronounced central effects, and much lower potency. Because of the development of more efficacious and 2-selective agonists, ephedrine is now used infrequently in treating asthma. Isoproterenol is a potent bronchodilator; when inhaled as a microaerosol from a pressurized canister, 80-120 mcg isoproterenol causes maximal bronchodilation within 5 minutes. Isoproterenol has a 60- to 90-minute duration of action. An increase in the asthma mortality rate that occurred in the United Kingdom in the mid 1960s was attributed to cardiac arrhythmias resulting from the use of high doses of inhaled isoproterenol. It is now rarely used for asthma. Figure 3. Bronchodilation is promoted by cAMP. Intracellular levels of cAMP can be increased by -adrenoceptor agonists, which increase the rate of its synthesis by adenylyl cyclase (AC); or by phosphodiesterase (PDE) inhibitors such as theophylline
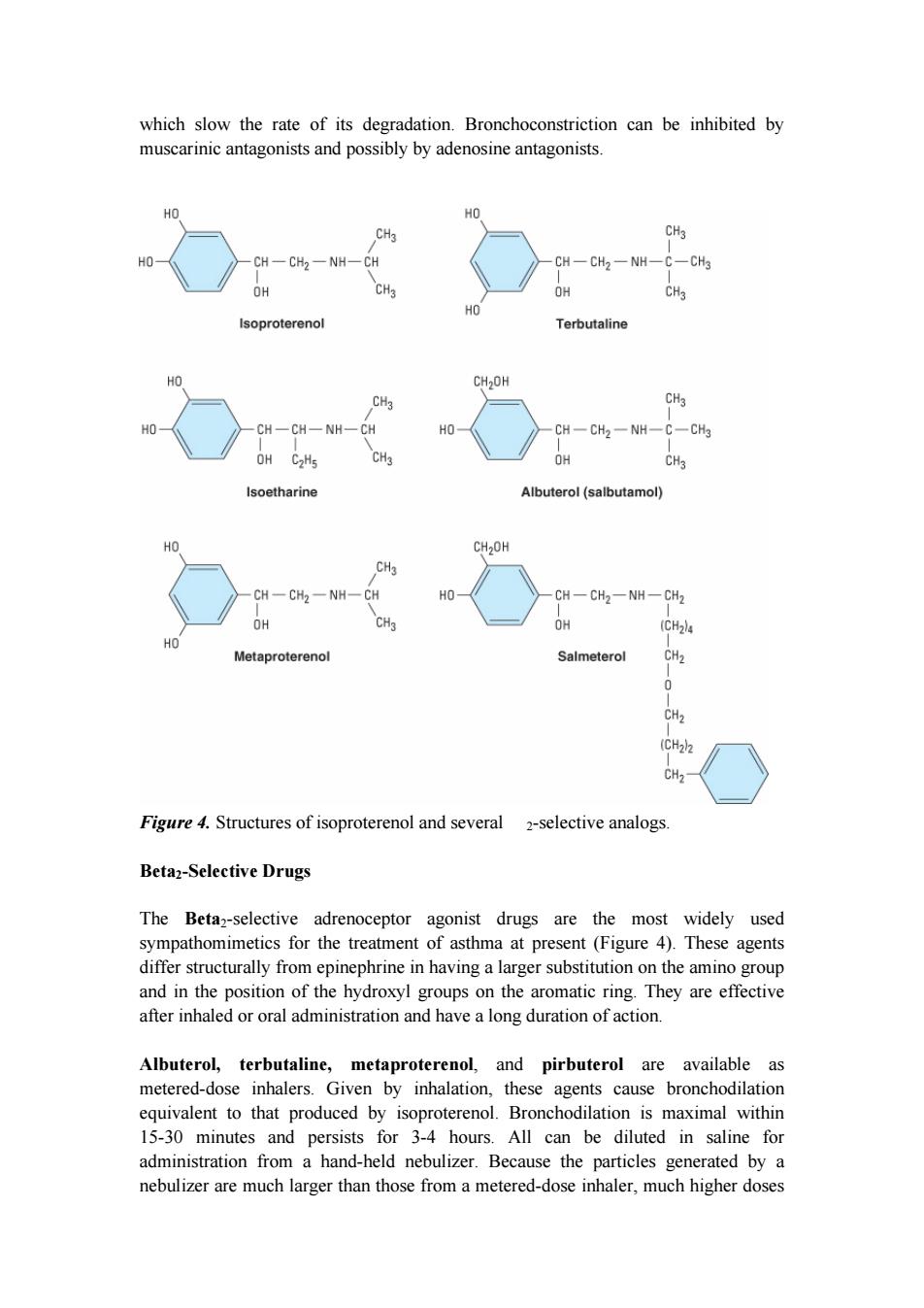
which slow the rate of its degradation.Bronchoconstriction can be inhibited by muscarinic antagonists and possibly by adenosine antagonists. HO HO CH3 CH3 CH一CH2一NH-CH CH-CH2-NH-C-CH3 OH CH3 OH CH3 HO Isoproterenol Terbutaline HO CH2OH CH3 CH3 HO CH-CH一NH-CH HO CH-CH2一NH-C-CH3 OH C2H5 CH3 OH CH3 Isoetharine Albuterol(salbutamol) HO CH2OH CH3 CH-CH2-NH-CH HO CH一CH2-NH-CH2 OH CH3 OH (CH24 HO Metaproterenol Salmeterol CH2 0 CH2 (CH22 CH2 Figure 4.Structures of isoproterenol and several 2-selective analogs. Beta2-Selective Drugs The Beta2-selective adrenoceptor agonist drugs are the most widely used sympathomimetics for the treatment of asthma at present(Figure 4).These agents differ structurally from epinephrine in having a larger substitution on the amino group and in the position of the hydroxyl groups on the aromatic ring.They are effective after inhaled or oral administration and have a long duration of action. Albuterol,terbutaline,metaproterenol,and pirbuterol are available as metered-dose inhalers.Given by inhalation,these agents cause bronchodilation equivalent to that produced by isoproterenol.Bronchodilation is maximal within 15-30 minutes and persists for 3-4 hours.All can be diluted in saline for administration from a hand-held nebulizer.Because the particles generated by a nebulizer are much larger than those from a metered-dose inhaler,much higher doses
which slow the rate of its degradation. Bronchoconstriction can be inhibited by muscarinic antagonists and possibly by adenosine antagonists. Figure 4. Structures of isoproterenol and several 2-selective analogs. Beta2-Selective Drugs The Beta2-selective adrenoceptor agonist drugs are the most widely used sympathomimetics for the treatment of asthma at present (Figure 4). These agents differ structurally from epinephrine in having a larger substitution on the amino group and in the position of the hydroxyl groups on the aromatic ring. They are effective after inhaled or oral administration and have a long duration of action. Albuterol, terbutaline, metaproterenol, and pirbuterol are available as metered-dose inhalers. Given by inhalation, these agents cause bronchodilation equivalent to that produced by isoproterenol. Bronchodilation is maximal within 15-30 minutes and persists for 3-4 hours. All can be diluted in saline for administration from a hand-held nebulizer. Because the particles generated by a nebulizer are much larger than those from a metered-dose inhaler, much higher doses

must be given(2.5-5.0 mg vs 100-400 mcg)but are no more effective.Nebulized therapy should thus be reserved for patients unable to coordinate inhalation from a metered-dose inhaler. Albuterol and terbutaline are also available in tablet form.One tablet two or three times daily is the usual regimen;the principal adverse effects of skeletal muscle tremor,nervousness,and occasional weakness may be reduced by starting the patient on half-strength tablets for the first 2 weeks of therapy,but this route of administration presents no advantage over inhaled treatment. Of these agents,only terbutaline is available for subcutaneous injection (0.25 mg). The indications for this route are similar to those for subcutaneous epinephrine severe asthma requiring emergency treatment when aerosolized therapy is not available or has been ineffective but it should be remembered that terbutaline's longer duration of action means that cumulative effects may be seen after repeated injections. A new generation of long-acting 2-selective agonists includes salmeterol and formoterol.Both drugs are potent selective 2 agonists that achieve their long duration of action(12 hours or more)as a result of high lipid solubility.This permits them to dissolve in the smooth muscle cell membrane in high concentrations or, possibly,attach to "mooring"molecules in the vicinity of the adrenoceptor.These drugs appear to interact with inhaled corticosteroids to improve asthma control.They are not recommended as the sole therapy for asthma. Toxicities The use of sympathomimetic agents by inhalation at first raised fears about possible cardiac arrhythmias and about hypoxemia acutely and tachyphylaxis or tolerance when given repeatedly.It is true that the vasodilating action of 2-agonist treatment may increase perfusion of poorly ventilated lung units,transiently decreasing arterial oxygen tension(PaO2).This effect is usually small,however,and may occur with any bronchodilator drug;the significance of such an effect depends on the initial PaOz of the patient.Administration of supplemental oxygen,routine in treatment of an acute severe attack of asthma,eliminates any concern over this effect.The other concern, that -agonist treatment may cause lethal cardiac arrhythmias appears unsubstantiated.In patients presenting for emergency treatment of severe asthma, irregularities in cardiac rhythm improve with the improvements in gas exchange effected by bronchodilator treatment. The concept that -agonist drugs cause worsening of clinical asthma by inducing tachyphylaxis to their own action remains unestablished.Most studies have shown only a small change in the bronchodilator response to stimulation after prolonged treatment with -agonist drugs,but some studies have shown a loss in the ability of
must be given (2.5-5.0 mg vs 100-400 mcg) but are no more effective. Nebulized therapy should thus be reserved for patients unable to coordinate inhalation from a metered-dose inhaler. Albuterol and terbutaline are also available in tablet form. One tablet two or three times daily is the usual regimen; the principal adverse effects of skeletal muscle tremor, nervousness, and occasional weakness may be reduced by starting the patient on half-strength tablets for the first 2 weeks of therapy, but this route of administration presents no advantage over inhaled treatment. Of these agents, only terbutaline is available for subcutaneous injection (0.25 mg). The indications for this route are similar to those for subcutaneous epinephrine severe asthma requiring emergency treatment when aerosolized therapy is not available or has been ineffective but it should be remembered that terbutaline's longer duration of action means that cumulative effects may be seen after repeated injections. A new generation of long-acting 2-selective agonists includes salmeterol and formoterol. Both drugs are potent selective 2 agonists that achieve their long duration of action (12 hours or more) as a result of high lipid solubility. This permits them to dissolve in the smooth muscle cell membrane in high concentrations or, possibly, attach to "mooring" molecules in the vicinity of the adrenoceptor. These drugs appear to interact with inhaled corticosteroids to improve asthma control. They are not recommended as the sole therapy for asthma. Toxicities The use of sympathomimetic agents by inhalation at first raised fears about possible cardiac arrhythmias and about hypoxemia acutely and tachyphylaxis or tolerance when given repeatedly. It is true that the vasodilating action of 2-agonist treatment may increase perfusion of poorly ventilated lung units, transiently decreasing arterial oxygen tension (PaO2). This effect is usually small, however, and may occur with any bronchodilator drug; the significance of such an effect depends on the initial PaO2 of the patient. Administration of supplemental oxygen, routine in treatment of an acute severe attack of asthma, eliminates any concern over this effect. The other concern, that -agonist treatment may cause lethal cardiac arrhythmias appears unsubstantiated. In patients presenting for emergency treatment of severe asthma, irregularities in cardiac rhythm improve with the improvements in gas exchange effected by bronchodilator treatment. The concept that -agonist drugs cause worsening of clinical asthma by inducing tachyphylaxis to their own action remains unestablished. Most studies have shown only a small change in the bronchodilator response to stimulation after prolonged treatment with -agonist drugs, but some studies have shown a loss in the ability of

-agonist treatment to inhibit the response to subsequent challenge with exercise, methacholine,or antigen challenge (referred to as a loss of bronchoprotective action). Fears that heavy use of -agonist inhalers could actually increase morbidity and mortality have not been borne out by careful epidemiologic investigations.Heavy use most often indicates that the patient should be receiving more effective prophylactic therapy with corticosteroids. Although it is true that 2-adrenoceptor agonists appear to be safe and effective bronchodilators for most patients,there is some evidence that the risk of adverse effects from chronic treatment with long-acting agonists may be greater for some individuals,possibly as a function of genetic variants for the receptor.Two retrospective and one prospective study have shown differences between patients homozygous for glycine versus arginine at the B-16 locus of the receptor.Among patients homozygous for arginine,a genotype found in 16%of the Caucasian population in the USA,but more commonly in African Americans,asthma control deteriorated with regular use of albuterol or salmeterol,whereas asthma control improved with this treatment among those homozygous for glycine at the same locus. These findings need to be replicated in larger studies,but it is tempting to speculate that a genetic variant may underlie the report of an increase in asthma mortality from regular use of a long-acting agonist in studies involving very large numbers of patients METHYLXANTHINE DRUGS Introduction The three important methylxanthines are theophylline,theobromine,and caffeine. Their major source is beverages(tea,cocoa,and coffee,respectively).The importance of theophylline as a therapeutic agent in the treatment of asthma has waned as the greater effectiveness of inhaled adrenoceptor agents for acute asthma and of inhaled anti-inflammatory agents for chronic asthma has been established,but theophylline's very low cost is an important advantage for economically disadvantaged patients in societies in which health care resources are limited. Chemistry As shown below,theophylline is 1,3-dimethylxanthine;theobromine is 3,7-dimethylxanthine;and caffeine is 1,3,7-trimethylxanthine.A theophylline preparation commonly used for therapeutic purposes is aminophylline,a theophylline-ethylenediamine complex.The pharmacokinetics of theophylline are discussed below.The metabolic products,partially demethylated xanthines(not uric acid),are excreted in the urine
-agonist treatment to inhibit the response to subsequent challenge with exercise, methacholine, or antigen challenge (referred to as a loss of bronchoprotective action). Fears that heavy use of -agonist inhalers could actually increase morbidity and mortality have not been borne out by careful epidemiologic investigations. Heavy use most often indicates that the patient should be receiving more effective prophylactic therapy with corticosteroids. Although it is true that 2-adrenoceptor agonists appear to be safe and effective bronchodilators for most patients, there is some evidence that the risk of adverse effects from chronic treatment with long-acting agonists may be greater for some individuals, possibly as a function of genetic variants for the receptor. Two retrospective and one prospective study have shown differences between patients homozygous for glycine versus arginine at the B-16 locus of the receptor. Among patients homozygous for arginine, a genotype found in 16% of the Caucasian population in the USA, but more commonly in African Americans, asthma control deteriorated with regular use of albuterol or salmeterol, whereas asthma control improved with this treatment among those homozygous for glycine at the same locus. These findings need to be replicated in larger studies, but it is tempting to speculate that a genetic variant may underlie the report of an increase in asthma mortality from regular use of a long-acting agonist in studies involving very large numbers of patients. METHYLXANTHINE DRUGS Introduction The three important methylxanthines are theophylline, theobromine, and caffeine. Their major source is beverages (tea, cocoa, and coffee, respectively). The importance of theophylline as a therapeutic agent in the treatment of asthma has waned as the greater effectiveness of inhaled adrenoceptor agents for acute asthma and of inhaled anti-inflammatory agents for chronic asthma has been established, but theophylline's very low cost is an important advantage for economically disadvantaged patients in societies in which health care resources are limited. Chemistry As shown below, theophylline is 1,3-dimethylxanthine; theobromine is 3,7-dimethylxanthine; and caffeine is 1,3,7-trimethylxanthine. A theophylline preparation commonly used for therapeutic purposes is aminophylline, a theophylline-ethylenediamine complex. The pharmacokinetics of theophylline are discussed below. The metabolic products, partially demethylated xanthines (not uric acid), are excreted in the urine