
Nonsteroidal Anti-Inflammatory Drugs,Disease-Modifying Antirheumatic Drugs,Nonopioid Analgesics,Drugs Used in Gout Daniel E.Furst,MD,Robert W.Ulrich,PharmD THE IMMUNE RESPONSE The immune response occurs when immunologically competent cells are activated in response to foreign organisms or antigenic substances liberated during the acute or chronic inflammatory response.The outcome of the immune response for the host may be beneficial,as when it causes invading organisms to be phagocytosed or neutralized.On the other hand,the outcome may be deleterious if it leads to chronic inflammation without resolution of the underlying injurious process.Chronic inflammation involves the release of a number of mediators that are not prominent in the acute response.One of the most important conditions involving these mediators is rheumatoid arthritis,in which chronic inflammation results in pain and destruction of bone and cartilage that can lead to severe disability and in which systemic changes occur that can result in shortening of life. The cell damage associated with inflammation acts on cell membranes to cause leukocytes to release lysosomal enzymes;arachidonic acid is then liberated from precursor compounds,and various eicosanoids are synthesized.As discussed in Chapter 18,the cyclooxygenase (COX)pathway of arachidonate metabolism produces prostaglandins,which have a variety of effects on blood vessels,on nerve endings,and on cells involved in inflammation.The discovery of cyclooxygenase isoforms (COX-1 and COX-2)led to the concepts that the constitutive COX-1 isoform tends to be homeostatic in function,while COX-2 is induced during inflammation and tends to facilitate the inflammatory response.On this basis,highly selective COX-2 inhibitors have been developed and marketed on the assumption that such selective inhibitors would be safer than nonselective COX-1 inhibitors but without loss of efficacy.The lipoxygenase pathway of arachidonate metabolism yields leukotrienes,which have a powerful chemotactic effect on eosinophils, neutrophils,and macrophages and promote bronchoconstriction and alterations in vascular permeability Kinins,neuropeptides,and histamine are also released at the site of tissue injury,as are complement components,cytokines,and other products of leukocytes and platelets.Stimulation of the neutrophil membranes produces oxygen-derived free radicals.Superoxide anion is formed by the reduction of molecular oxygen,which may stimulate the production of other reactive molecules such as hydrogen peroxide and hydroxyl radicals.The interaction of these substances with arachidonic acid results in the generation of chemotactic substances,thus perpetuating the inflammatory process. THERAPEUTIC STRATEGIES
Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout Daniel E. Furst, MD, & Robert W. Ulrich, PharmD THE IMMUNE RESPONSE The immune response occurs when immunologically competent cells are activated in response to foreign organisms or antigenic substances liberated during the acute or chronic inflammatory response. The outcome of the immune response for the host may be beneficial, as when it causes invading organisms to be phagocytosed or neutralized. On the other hand, the outcome may be deleterious if it leads to chronic inflammation without resolution of the underlying injurious process. Chronic inflammation involves the release of a number of mediators that are not prominent in the acute response. One of the most important conditions involving these mediators is rheumatoid arthritis, in which chronic inflammation results in pain and destruction of bone and cartilage that can lead to severe disability and in which systemic changes occur that can result in shortening of life. The cell damage associated with inflammation acts on cell membranes to cause leukocytes to release lysosomal enzymes; arachidonic acid is then liberated from precursor compounds, and various eicosanoids are synthesized. As discussed in Chapter 18, the cyclooxygenase (COX) pathway of arachidonate metabolism produces prostaglandins, which have a variety of effects on blood vessels, on nerve endings, and on cells involved in inflammation. The discovery of cyclooxygenase isoforms (COX-1 and COX-2) led to the concepts that the constitutive COX-1 isoform tends to be homeostatic in function, while COX-2 is induced during inflammation and tends to facilitate the inflammatory response. On this basis, highly selective COX-2 inhibitors have been developed and marketed on the assumption that such selective inhibitors would be safer than nonselective COX-1 inhibitors but without loss of efficacy. The lipoxygenase pathway of arachidonate metabolism yields leukotrienes, which have a powerful chemotactic effect on eosinophils, neutrophils, and macrophages and promote bronchoconstriction and alterations in vascular permeability. Kinins, neuropeptides, and histamine are also released at the site of tissue injury, as are complement components, cytokines, and other products of leukocytes and platelets. Stimulation of the neutrophil membranes produces oxygen-derived free radicals. Superoxide anion is formed by the reduction of molecular oxygen, which may stimulate the production of other reactive molecules such as hydrogen peroxide and hydroxyl radicals. The interaction of these substances with arachidonic acid results in the generation of chemotactic substances, thus perpetuating the inflammatory process. THERAPEUTIC STRATEGIES

The treatment of patients with inflammation involves two primary goals:first,the relief of pain,which is often the presenting symptom and the major continuing complaint of the patient;and second,the slowing or%in theoryarrest of the tissue-damaging process.In rheumatoid arthritis,response to therapy can be quantitated by means of the American College of Rheumatology scoring system values ACR20,ACR50,and ACR70,which denote the percentage of patients showing an improvement of 20%,50%,or 70%in a global assessment of signs and symptoms. Reduction of inflammation with nonsteroidal anti-inflammatory drugs (NSAIDs) often results in relief of pain for significant periods.Furthermore,most of the nonopioid analgesics (aspirin,etc)also have anti-inflammatory effects,so they are appropriate for the treatment of both acute and chronic inflammatory conditions. The glucocorticoids also have powerful anti-inflammatory effects and when first introduced were considered to be the ultimate answer to the treatment of inflammatory arthritis.Although there are increasing data that low-dose corticosteroids have disease-modifying properties,the toxicity associated with chronic corticosteroid therapy usually limits their use except in the control of acute flare-ups of joint disease.The NSAIDs continue to have a significant role in the long-term treatment of arthritis. Another important group of agents is characterized as disease-modifying antirheumatic drugs (DMARDs).They slow the bone damage associated with rheumatoid arthritis and are thought to affect more basic inflammatory mechanisms than do the NSAIDs.They may also be more toxic than the nonsteroidal anti-inflammatory agents. I.NONSTEROIDAL ANTI-INFLAMMATORY DRUGS Introduction Salicylates and other similar agents used to treat rheumatic disease share the capacity to suppress the signs and symptoms of inflammation.These drugs also exert antipyretic and analgesic effects,but it is their anti-inflammatory properties that make them most useful in the management of disorders in which pain is related to the intensity of the inflammatory process. Although all NSAIDs are not approved by the Food and Drug Administration(FDA) for the whole range of rheumatic diseases,all are probably effective in rheumatoid arthritis,seronegative spondyloarthropathies (eg,psoriatic arthritis and arthritis associated with inflammatory bowel disease),osteoarthritis,localized musculoskeletal
The treatment of patients with inflammation involves two primary goals: first, the relief of pain, which is often the presenting symptom and the major continuing complaint of the patient; and second, the slowing or¾in theory¾arrest of the tissue-damaging process. In rheumatoid arthritis, response to therapy can be quantitated by means of the American College of Rheumatology scoring system values ACR20, ACR50, and ACR70, which denote the percentage of patients showing an improvement of 20%, 50%, or 70% in a global assessment of signs and symptoms. Reduction of inflammation with nonsteroidal anti-inflammatory drugs (NSAIDs) often results in relief of pain for significant periods. Furthermore, most of the nonopioid analgesics (aspirin, etc) also have anti-inflammatory effects, so they are appropriate for the treatment of both acute and chronic inflammatory conditions. The glucocorticoids also have powerful anti-inflammatory effects and when first introduced were considered to be the ultimate answer to the treatment of inflammatory arthritis. Although there are increasing data that low-dose corticosteroids have disease-modifying properties, the toxicity associated with chronic corticosteroid therapy usually limits their use except in the control of acute flare-ups of joint disease. The NSAIDs continue to have a significant role in the long-term treatment of arthritis. Another important group of agents is characterized as disease-modifying antirheumatic drugs (DMARDs). They slow the bone damage associated with rheumatoid arthritis and are thought to affect more basic inflammatory mechanisms than do the NSAIDs. They may also be more toxic than the nonsteroidal anti-inflammatory agents. I. NONSTEROIDAL ANTI-INFLAMMATORY DRUGS Introduction Salicylates and other similar agents used to treat rheumatic disease share the capacity to suppress the signs and symptoms of inflammation. These drugs also exert antipyretic and analgesic effects, but it is their anti-inflammatory properties that make them most useful in the management of disorders in which pain is related to the intensity of the inflammatory process. Although all NSAIDs are not approved by the Food and Drug Administration (FDA) for the whole range of rheumatic diseases, all are probably effective in rheumatoid arthritis, seronegative spondyloarthropathies (eg, psoriatic arthritis and arthritis associated with inflammatory bowel disease), osteoarthritis, localized musculoskeletal

syndromes (eg,sprains and strains,low back pain),and gout (except tolmetin,which appears to be ineffective in gout).Since aspirin,the original NSAID,has a number of adverse effects,many other NSAIDs have been developed in attempts to improve upon aspirin's efficacy and decrease its toxicity. Chemistry Pharmacokinetics The NSAIDs are grouped in several chemical classes,some of which are shown in Figure 36-1.This chemical diversity yields a broad range of pharmacokinetic characteristics.Although there are many differences in the kinetics of NSAIDs,they have some general properties in common.All but one of the NSAIDs are weak organic acids as given;the exception,nabumetone,is a ketone prodrug that is metabolized to the acidic active drug.Most of these drugs are well absorbed,and food does not substantially change their bioavailability.Most of the NSAIDs are highly metabolized,some by phase I followed by phase II mechanisms and others by direct glucuronidation (phase II)alone.NSAID metabolism proceeds,in large part,by way of the CYP3A or CYP2C families of P450 enzymes in the liver.While renal excretion is the most important route for final elimination,nearly all undergo varying degrees of biliary excretion and reabsorption (enterohepatic circulation).In fact,the degree of lower gastrointestinal tract irritation correlates with the amount of enterohepatic circulation.Most of the NSAIDs are highly protein-bound (98%),usually to albumin.Some of the NSAIDs (eg,ibuprofen)are racemic mixtures,while one, naproxen,is provided as a single enantiomer and a few have no chiral center (eg, diclofenac). All NSAIDs can be found in synovial fluid after repeated dosing.Drugs with short half-lives remain in the joints longer than would be predicted from their half-lives, while drugs with longer half-lives disappear from the synovial fluid at a rate proportionate to their half-lives
syndromes (eg, sprains and strains, low back pain), and gout (except tolmetin, which appears to be ineffective in gout). Since aspirin, the original NSAID, has a number of adverse effects, many other NSAIDs have been developed in attempts to improve upon aspirin's efficacy and decrease its toxicity. Chemistry & Pharmacokinetics The NSAIDs are grouped in several chemical classes, some of which are shown in Figure 36-1. This chemical diversity yields a broad range of pharmacokinetic characteristics. Although there are many differences in the kinetics of NSAIDs, they have some general properties in common. All but one of the NSAIDs are weak organic acids as given; the exception, nabumetone, is a ketone prodrug that is metabolized to the acidic active drug. Most of these drugs are well absorbed, and food does not substantially change their bioavailability. Most of the NSAIDs are highly metabolized, some by phase I followed by phase II mechanisms and others by direct glucuronidation (phase II) alone. NSAID metabolism proceeds, in large part, by way of the CYP3A or CYP2C families of P450 enzymes in the liver. While renal excretion is the most important route for final elimination, nearly all undergo varying degrees of biliary excretion and reabsorption (enterohepatic circulation). In fact, the degree of lower gastrointestinal tract irritation correlates with the amount of enterohepatic circulation. Most of the NSAIDs are highly protein-bound (~ 98%), usually to albumin. Some of the NSAIDs (eg, ibuprofen) are racemic mixtures, while one, naproxen, is provided as a single enantiomer and a few have no chiral center (eg, diclofenac). All NSAIDs can be found in synovial fluid after repeated dosing. Drugs with short half-lives remain in the joints longer than would be predicted from their half-lives, while drugs with longer half-lives disappear from the synovial fluid at a rate proportionate to their half-lives
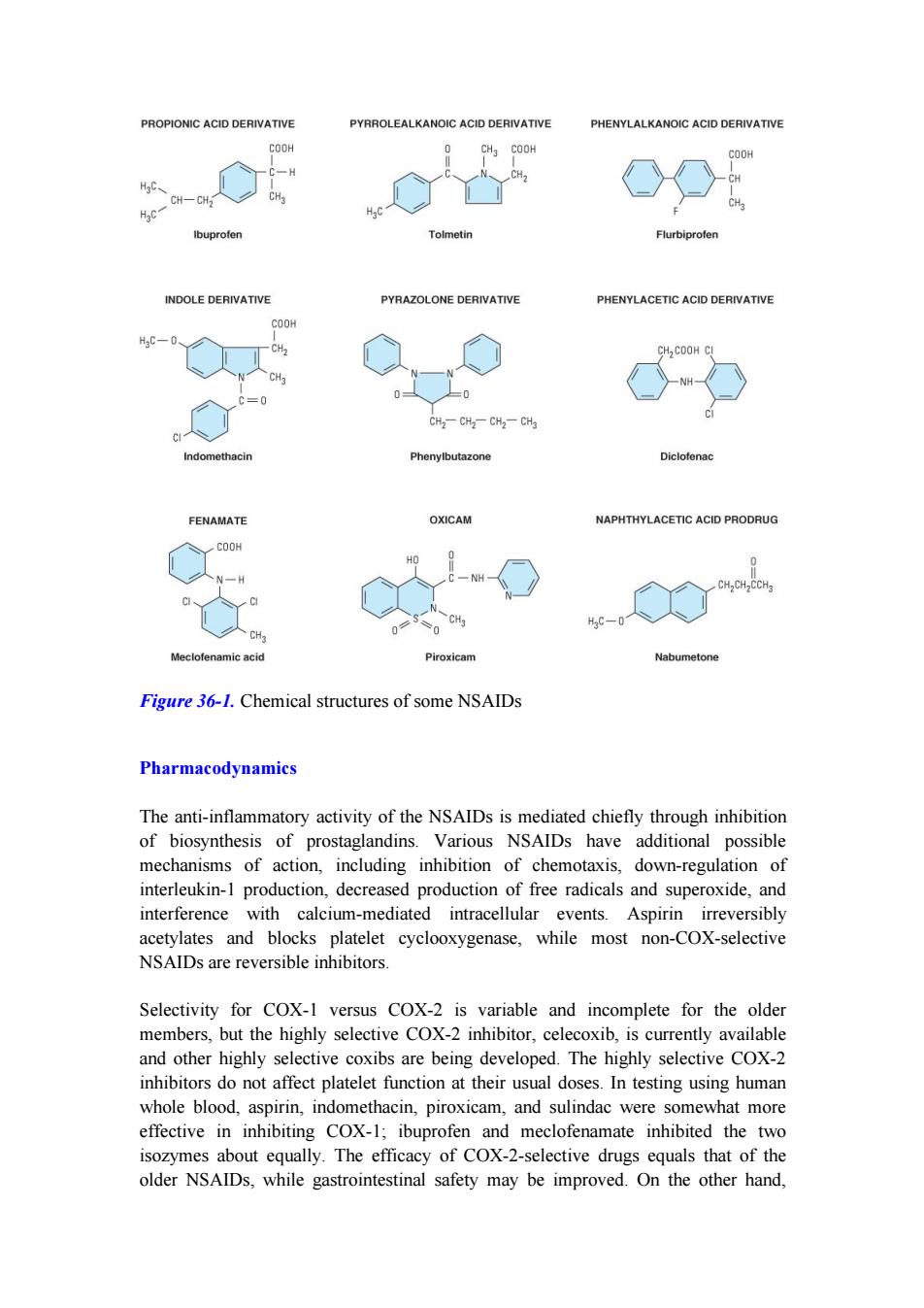
PROPIONIC ACID DERIVATIVE PYRROLEALKANOIC ACID DERIVATIVE PHENYLALKANOIC ACID DERIVATIVE COOH CH3 COOH COOH CH HgC、 CH-CH CH 作c CH Ibuprofen Tolmetin Flurbiprofen INDOLE DERIVATIVE PYRAZOLONE DERIVATIVE PHENYLACETIC ACID DERIVATIVE COOH H3C-0 CH2 CH COOH CI CH2-CH2-CH2-CHa CI Indomethacin Phenylbutazone Diclofenac FENAMATE OXICAM NAPHTHYLACETIC ACID PRODRUG COOH N-H CH,CH CCH3 HaC-0 Meclofenamic acid Piroxicam Nabumetone Figure 36-1.Chemical structures of some NSAIDs Pharmacodynamics The anti-inflammatory activity of the NSAIDs is mediated chiefly through inhibition of biosynthesis of prostaglandins.Various NSAIDs have additional possible mechanisms of action,including inhibition of chemotaxis,down-regulation of interleukin-1 production,decreased production of free radicals and superoxide,and interference with calcium-mediated intracellular events.Aspirin irreversibly acetylates and blocks platelet cyclooxygenase,while most non-COX-selective NSAIDs are reversible inhibitors. Selectivity for COX-1 versus COX-2 is variable and incomplete for the older members,but the highly selective COX-2 inhibitor,celecoxib,is currently available and other highly selective coxibs are being developed.The highly selective COX-2 inhibitors do not affect platelet function at their usual doses.In testing using human whole blood,aspirin,indomethacin,piroxicam,and sulindac were somewhat more effective in inhibiting COX-1;ibuprofen and meclofenamate inhibited the two isozymes about equally.The efficacy of COX-2-selective drugs equals that of the older NSAIDs,while gastrointestinal safety may be improved.On the other hand
Figure 36-1. Chemical structures of some NSAIDs Pharmacodynamics The anti-inflammatory activity of the NSAIDs is mediated chiefly through inhibition of biosynthesis of prostaglandins. Various NSAIDs have additional possible mechanisms of action, including inhibition of chemotaxis, down-regulation of interleukin-1 production, decreased production of free radicals and superoxide, and interference with calcium-mediated intracellular events. Aspirin irreversibly acetylates and blocks platelet cyclooxygenase, while most non-COX-selective NSAIDs are reversible inhibitors. Selectivity for COX-1 versus COX-2 is variable and incomplete for the older members, but the highly selective COX-2 inhibitor, celecoxib, is currently available and other highly selective coxibs are being developed. The highly selective COX-2 inhibitors do not affect platelet function at their usual doses. In testing using human whole blood, aspirin, indomethacin, piroxicam, and sulindac were somewhat more effective in inhibiting COX-1; ibuprofen and meclofenamate inhibited the two isozymes about equally. The efficacy of COX-2-selective drugs equals that of the older NSAIDs, while gastrointestinal safety may be improved. On the other hand

highly selective COX-2 inhibitors may increase the incidence of edema and hypertension.As of August 2006,celecoxib is the only COX-2 inhibitor marketed in the USA.Rofecoxib and valdecoxib,two previously marketed,highly selective COX-2 inhibitors,have been withdrawn from the market due to their association with increased cardiovascular thrombotic events.In early 2005 a joint meeting of the Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee conducted by the FDA convened to assess the risk and give recommendations concerning the COX-2 inhibitors and other NSAIDs.It was concluded that there was not sufficient evidence to withdraw the COX-2 inhibitors but "black box"warnings concerning the cardiovascular risks were added to the product label.Additionally,it was recommended that all other NSAID product labels be revised to include cardiovascular risks.Presently,the future of the COX-2 inhibitors is unclear. The NSAIDs decrease the sensitivity of vessels to bradykinin and histamine,affect lymphokine production from T lymphocytes,and reverse the vasodilation of inflammation.To varying degrees,all newer NSAIDs are analgesic, anti-inflammatory,and antipyretic,and all(except the COX-2-selective agents and the nonacetylated salicylates)inhibit platelet aggregation.NSAIDs are all gastric irritants as well,although as a group the newer agents tend to cause less gastric irritation than aspirin.Nephrotoxicity has been observed for all of the drugs for which extensive experience has been reported,and hepatotoxicity can also occur with any NSAID Nephrotoxicity is due,in part,to interference with the autoregulation of renal blood flow,which is modulated by prostaglandins. Although these drugs effectively inhibit inflammation,there is no evidence that%in contrast to drugs such as methotrexate and goldthey alter the course of an arthritic disorder. Several NSAIDs (including aspirin)appear to reduce the incidence of colon cancer when taken chronically.Several large epidemiologic studies have shown a 50% reduction in relative risk when the drugs are taken for 5 years or longer.The mechanism for this protective effect is unclear. ASPIRIN Introduction Aspirin's long use and availability without prescription diminishes its glamour compared with that of the newer NSAIDs.Aspirin is now rarely used as an anti-inflammatory medication;it has been replaced by ibuprofen and naproxen,since they are effective,are also available over the counter,and have good to excellent safety records
highly selective COX-2 inhibitors may increase the incidence of edema and hypertension. As of August 2006, celecoxib is the only COX-2 inhibitor marketed in the USA. Rofecoxib and valdecoxib, two previously marketed, highly selective COX-2 inhibitors, have been withdrawn from the market due to their association with increased cardiovascular thrombotic events. In early 2005 a joint meeting of the Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee conducted by the FDA convened to assess the risk and give recommendations concerning the COX-2 inhibitors and other NSAIDs. It was concluded that there was not sufficient evidence to withdraw the COX-2 inhibitors but "black box" warnings concerning the cardiovascular risks were added to the product label. Additionally, it was recommended that all other NSAID product labels be revised to include cardiovascular risks. Presently, the future of the COX-2 inhibitors is unclear. The NSAIDs decrease the sensitivity of vessels to bradykinin and histamine, affect lymphokine production from T lymphocytes, and reverse the vasodilation of inflammation. To varying degrees, all newer NSAIDs are analgesic, anti-inflammatory, and antipyretic, and all (except the COX-2-selective agents and the nonacetylated salicylates) inhibit platelet aggregation. NSAIDs are all gastric irritants as well, although as a group the newer agents tend to cause less gastric irritation than aspirin. Nephrotoxicity has been observed for all of the drugs for which extensive experience has been reported, and hepatotoxicity can also occur with any NSAID. Nephrotoxicity is due, in part, to interference with the autoregulation of renal blood flow, which is modulated by prostaglandins. Although these drugs effectively inhibit inflammation, there is no evidence that¾in contrast to drugs such as methotrexate and gold¾they alter the course of an arthritic disorder. Several NSAIDs (including aspirin) appear to reduce the incidence of colon cancer when taken chronically. Several large epidemiologic studies have shown a 50% reduction in relative risk when the drugs are taken for 5 years or longer. The mechanism for this protective effect is unclear. ASPIRIN Introduction Aspirin's long use and availability without prescription diminishes its glamour compared with that of the newer NSAIDs. Aspirin is now rarely used as an anti-inflammatory medication; it has been replaced by ibuprofen and naproxen, since they are effective, are also available over the counter, and have good to excellent safety records

Pharmacokinetics Salicylic acid is a simple organic acid with a pKa of 3.0.Aspirin(acetylsalicylic acid; ASA)has a pKa of 3.5.Sodium salicylate and aspirin (Figure 36-2)are equally effective anti-inflammatory drugs,though aspirin may be more effective as an analgesic.The salicylates are rapidly absorbed from the stomach and upper small intestine,yielding a peak plasma salicylate level within 1-2 hours.Aspirin is absorbed as such and is rapidly hydrolyzed (serum half-life 15 minutes)to acetic acid and salicylate by esterases in tissue and blood.Salicylate is bound to albumin,but the binding and metabolism of salicylates are saturable so that the unbound fraction increases as total concentration increases.Beyond a total body load of 600 mg, increases in salicylate dosage increase salicylate concentration disproportionately.As doses of aspirin increase,salicylate elimination half-life increases from 3-5 hours(for 600 mg/d dosage)to 12-16 hours (dosage 3.6 g/d).Alkalinization of the urine increases the rate of excretion of free salicylate and its water-soluble conjugates. 0-Na CH OH Aspirin 0 Sodium salicylate -0 HO-C-CH3 OH Acetic acid Salicylate Conjugation with Conjugation glucuronic acid Oxidation with glycine H 0 Ester and ether -N-CH2-COOH Free HO -0H glucuronides salicylate OH OH Salicyluric acid Gentisic acid (1%) Figure 36-2.Structure and metabolism of the salicylates.(Modified and reproduced, with permission,from Meyers FH,Jawetz E,Goldfien A:Review of Medical Pharmacology,7th ed.McGraw-Hill,1980.) Mechanisms of Action A.ANTI-INFLAMMATORY EFFECTS Aspirin is a nonselective inhibitor of both COX isoforms(Figure 36-3),but salicylate is much less effective in inhibiting either isoform.Nonacetylated salicylates may work as oxygen radical scavengers.Aspirin irreversibly inhibits COX and inhibits platelet aggregation,while nonacetylated salicylates do not
Pharmacokinetics Salicylic acid is a simple organic acid with a pKa of 3.0. Aspirin (acetylsalicylic acid; ASA) has a pKa of 3.5. Sodium salicylate and aspirin (Figure 36-2) are equally effective anti-inflammatory drugs, though aspirin may be more effective as an analgesic. The salicylates are rapidly absorbed from the stomach and upper small intestine, yielding a peak plasma salicylate level within 1-2 hours. Aspirin is absorbed as such and is rapidly hydrolyzed (serum half-life 15 minutes) to acetic acid and salicylate by esterases in tissue and blood. Salicylate is bound to albumin, but the binding and metabolism of salicylates are saturable so that the unbound fraction increases as total concentration increases. Beyond a total body load of 600 mg, increases in salicylate dosage increase salicylate concentration disproportionately. As doses of aspirin increase, salicylate elimination half-life increases from 3-5 hours (for 600 mg/d dosage) to 12-16 hours (dosage > 3.6 g/d). Alkalinization of the urine increases the rate of excretion of free salicylate and its water-soluble conjugates. Figure 36-2. Structure and metabolism of the salicylates. (Modified and reproduced, with permission, from Meyers FH, Jawetz E, Goldfien A: Review of Medical Pharmacology, 7th ed. McGraw-Hill, 1980.) Mechanisms of Action A. ANTI-INFLAMMATORY EFFECTS Aspirin is a nonselective inhibitor of both COX isoforms (Figure 36-3), but salicylate is much less effective in inhibiting either isoform. Nonacetylated salicylates may work as oxygen radical scavengers. Aspirin irreversibly inhibits COX and inhibits platelet aggregation, while nonacetylated salicylates do not
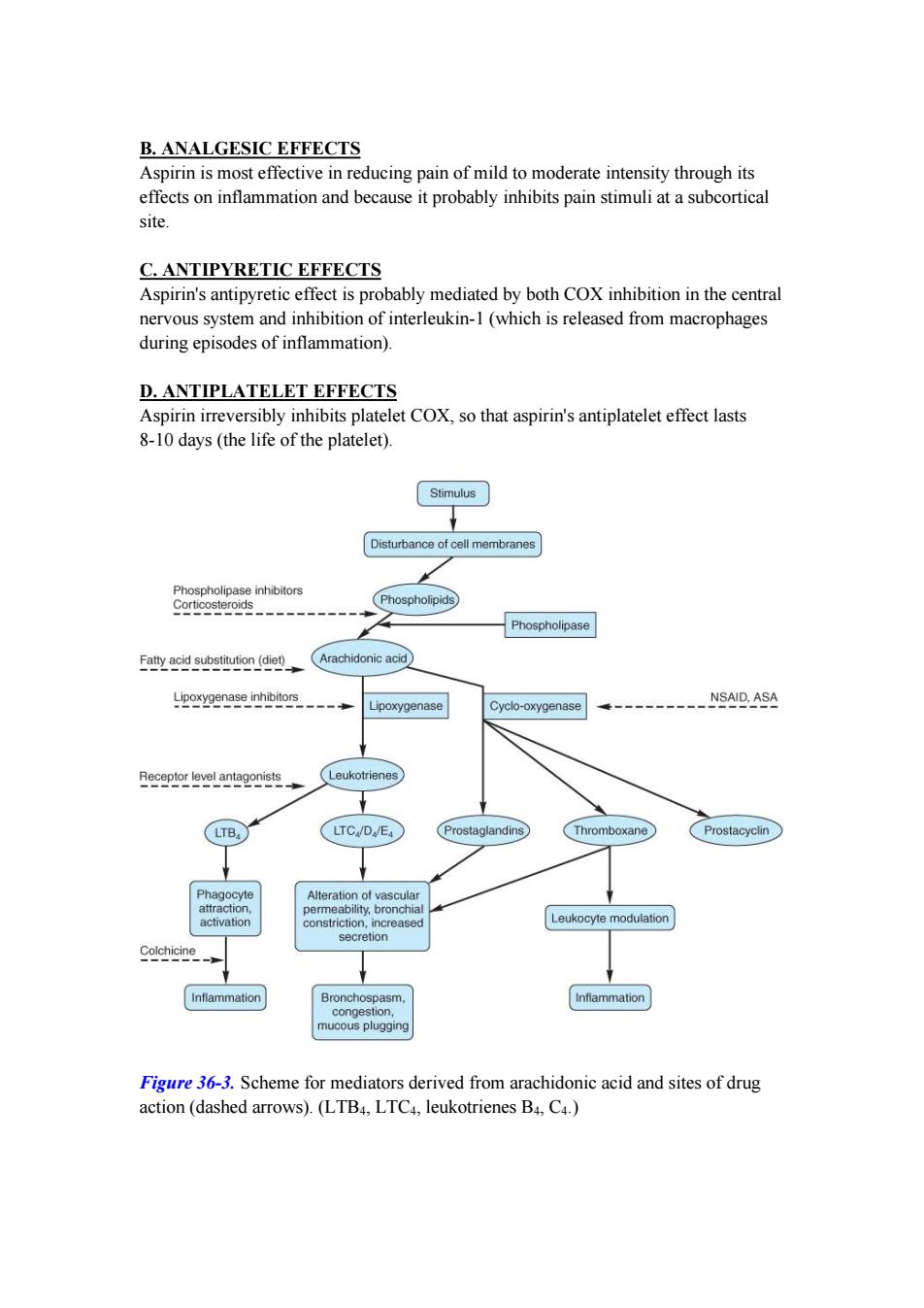
B.ANALGESIC EFFECTS Aspirin is most effective in reducing pain of mild to moderate intensity through its effects on inflammation and because it probably inhibits pain stimuli at a subcortical site. C.ANTIPYRETIC EFFECTS Aspirin's antipyretic effect is probably mediated by both COX inhibition in the central nervous system and inhibition of interleukin-1(which is released from macrophages during episodes of inflammation). D.ANTIPLATELET EFFECTS Aspirin irreversibly inhibits platelet COX,so that aspirin's antiplatelet effect lasts 8-10 days(the life of the platelet). Stimulus Disturbance of cell membranes Phospholipase inhibitors Corticosteroids Phospholipids Phospholipase Fatty acid substitution(diet) Arachidonic acid Lipoxygenase inhibitors NSAID,ASA Lipoxygenase Cyclo-oxygenase Receptor level antagonists Leukotrienes LTB, LTC/D/E4 Prostaglandins Thromboxane Prostacyclin Phagocyte Alteration of vascular attraction, permeability,bronchial activation constriction,increased Leukocyte modulation secretion Colchicine Inflammation Bronchospasm, Inflammation congestion, mucous plugging Figure 36-3.Scheme for mediators derived from arachidonic acid and sites of drug action(dashed arrows).(LTB4,LTC4,leukotrienes B4,C4.)
B. ANALGESIC EFFECTS Aspirin is most effective in reducing pain of mild to moderate intensity through its effects on inflammation and because it probably inhibits pain stimuli at a subcortical site. C. ANTIPYRETIC EFFECTS Aspirin's antipyretic effect is probably mediated by both COX inhibition in the central nervous system and inhibition of interleukin-1 (which is released from macrophages during episodes of inflammation). D. ANTIPLATELET EFFECTS Aspirin irreversibly inhibits platelet COX, so that aspirin's antiplatelet effect lasts 8-10 days (the life of the platelet). Figure 36-3. Scheme for mediators derived from arachidonic acid and sites of drug action (dashed arrows). (LTB4, LTC4, leukotrienes B4, C4.)

Clinical Uses A.ANALGESIA,ANTIPYRESIS,AND ANTI-INFLAMMATORY EFFECTS Aspirin is employed for mild to moderate pain of varied origin but is not effective for severe visceral pain.Aspirin and other NSAIDs have been combined with opioid analgesics for treatment of cancer pain,where their anti-inflammatory effects act synergistically with the opioids to enhance analgesia.High-dose salicylates are effective for treatment of rheumatic fever,rheumatoid arthritis,and other inflammatory joint conditions. B.OTHER EFFECTS Aspirin decreases the incidence of transient ischemic attacks,unstable angina, coronary artery thrombosis with myocardial infarction,and thrombosis after coronary artery bypass grafting(see Chapter 34). Epidemiologic studies suggest that long-term use of aspirin at low dosage is associated with a lower incidence of colon cancer,possibly related to its COX-inhibiting effects. Dosage The optimal analgesic or antipyretic dose of aspirin is less than the 0.6-0.65 g oral dose commonly used.The anti-inflammatory dose for children is 50-75 mg/kg/d in divided doses and the average starting anti-inflammatory dose for adults is 45 mg/kg/d in divided dose. Adverse Effects At the usual dosage,aspirin's main adverse effects are gastric upset (intolerance)and gastric and duodenal ulcers;hepatotoxicity,asthma,rashes,and renal toxicity occur less frequently.A dose-related increase in fecal blood loss is routinely associated with aspirin administration,although some mucosal adaptation occurs in many patients,so that blood loss declines back to baseline over 4-6 weeks. With higher doses,patients may experience salicylismvomiting,tinnitus,decreased hearing,and vertigoreversible by reducing the dosage.Still larger doses of salicylates cause hyperpnea through a direct effect on the medulla.At toxic salicylate levels,respiratory alkalosis followed by metabolic acidosis(salicylate accumulation). respiratory depression,and even cardiotoxicity and glucose intolerance can occur. Like other NSAIDs,aspirin can cause elevation of liver enzymes (a frequent but mild effect),hepatitis(rare),decreased renal function,bleeding,rashes,and asthma. The antiplatelet action of aspirin contraindicates its use by patients with hemophilia. Although previously not recommended during pregnancy,aspirin may be valuable in
Clinical Uses A. ANALGESIA, ANTIPYRESIS, AND ANTI-INFLAMMATORY EFFECTS Aspirin is employed for mild to moderate pain of varied origin but is not effective for severe visceral pain. Aspirin and other NSAIDs have been combined with opioid analgesics for treatment of cancer pain, where their anti-inflammatory effects act synergistically with the opioids to enhance analgesia. High-dose salicylates are effective for treatment of rheumatic fever, rheumatoid arthritis, and other inflammatory joint conditions. B. OTHER EFFECTS Aspirin decreases the incidence of transient ischemic attacks, unstable angina, coronary artery thrombosis with myocardial infarction, and thrombosis after coronary artery bypass grafting (see Chapter 34). Epidemiologic studies suggest that long-term use of aspirin at low dosage is associated with a lower incidence of colon cancer, possibly related to its COX-inhibiting effects. Dosage The optimal analgesic or antipyretic dose of aspirin is less than the 0.6-0.65 g oral dose commonly used. The anti-inflammatory dose for children is 50-75 mg/kg/d in divided doses and the average starting anti-inflammatory dose for adults is 45 mg/kg/d in divided dose. Adverse Effects At the usual dosage, aspirin's main adverse effects are gastric upset (intolerance) and gastric and duodenal ulcers; hepatotoxicity, asthma, rashes, and renal toxicity occur less frequently. A dose-related increase in fecal blood loss is routinely associated with aspirin administration, although some mucosal adaptation occurs in many patients, so that blood loss declines back to baseline over 4-6 weeks. With higher doses, patients may experience salicylism¾vomiting, tinnitus, decreased hearing, and vertigo¾reversible by reducing the dosage. Still larger doses of salicylates cause hyperpnea through a direct effect on the medulla. At toxic salicylate levels, respiratory alkalosis followed by metabolic acidosis (salicylate accumulation), respiratory depression, and even cardiotoxicity and glucose intolerance can occur. Like other NSAIDs, aspirin can cause elevation of liver enzymes (a frequent but mild effect), hepatitis (rare), decreased renal function, bleeding, rashes, and asthma. The antiplatelet action of aspirin contraindicates its use by patients with hemophilia. Although previously not recommended during pregnancy, aspirin may be valuable in

treating preeclampsia-eclampsia Salicylate overdosage constitutes a medical emergency and requires hospitalization. NONACETYLATED SALICYLATES Introduction These drugs include magnesium choline salicylate,sodium salicylate,and salicylsalicylate.All nonacetylated salicylates are effective anti-inflammatory drugs, although they may be less effective analgesics than aspirin.Because they are much less effective than aspirin as COX inhibitors,they may be preferable when COX inhibition is undesirable,such as in patients with asthma,those with bleeding tendencies,and even (under close supervision)those with renal dysfunction. The nonacetylated salicylates are administered in the same dosage as aspirin and can be monitored using serum salicylate measurements. COX-2 Selective Inhibitors COX-2 selective inhibitors,or coxibs,were developed in an attempt to inhibit prostaglandin synthesis by the COX-2 isoenzyme induced at sites of inflammation without affecting the action of the constitutively active "housekeeping"COX-1 isoenzyme found in the gastrointestinal tract,kidneys,and platelets.Coxibs selectively bind to and block the active site of the COX-2 enzyme much more effectively than that of COX-1.COX-2 inhibitors have analgesic,antipyretic,and anti-inflammatory effects similar to those of nonselective NSAIDs but with an approximate halving of gastrointestinal adverse effects.Likewise,COX-2 inhibitors at usual doses have been shown to have no impact on platelet aggregation,which is mediated by the COX-1 isoenzyme.As a result,COX-2 inhibitors do not offer the cardioprotective effects of traditional nonselective NSAIDs,which has resulted in some patients taking low-dose aspirin in addition to a coxib regimen to maintain this effect.Unfortunately,because COX-2 is constitutively active within the kidney, recommended doses of COX-2 inhibitors cause renal toxicities similar to those associated with traditional NSAIDs.Clinical data have suggested a higher incidence of cardiovascular thrombotic events associated with COX-2 inhibitors such as rofecoxib and valdecoxib,resulting in their withdrawal from the market. 1.Celecoxib Celecoxib is a selective COX-2 inhibitorabout 10-20 times more selective for COX-2 than for COX-1
treating preeclampsia-eclampsia. Salicylate overdosage constitutes a medical emergency and requires hospitalization. NONACETYLATED SALICYLATES Introduction These drugs include magnesium choline salicylate, sodium salicylate, and salicylsalicylate. All nonacetylated salicylates are effective anti-inflammatory drugs, although they may be less effective analgesics than aspirin. Because they are much less effective than aspirin as COX inhibitors, they may be preferable when COX inhibition is undesirable, such as in patients with asthma, those with bleeding tendencies, and even (under close supervision) those with renal dysfunction. The nonacetylated salicylates are administered in the same dosage as aspirin and can be monitored using serum salicylate measurements. COX-2 Selective Inhibitors COX-2 selective inhibitors, or coxibs, were developed in an attempt to inhibit prostaglandin synthesis by the COX-2 isoenzyme induced at sites of inflammation without affecting the action of the constitutively active "housekeeping" COX-1 isoenzyme found in the gastrointestinal tract, kidneys, and platelets. Coxibs selectively bind to and block the active site of the COX-2 enzyme much more effectively than that of COX-1. COX-2 inhibitors have analgesic, antipyretic, and anti-inflammatory effects similar to those of nonselective NSAIDs but with an approximate halving of gastrointestinal adverse effects. Likewise, COX-2 inhibitors at usual doses have been shown to have no impact on platelet aggregation, which is mediated by the COX-1 isoenzyme. As a result, COX-2 inhibitors do not offer the cardioprotective effects of traditional nonselective NSAIDs, which has resulted in some patients taking low-dose aspirin in addition to a coxib regimen to maintain this effect. Unfortunately, because COX-2 is constitutively active within the kidney, recommended doses of COX-2 inhibitors cause renal toxicities similar to those associated with traditional NSAIDs. Clinical data have suggested a higher incidence of cardiovascular thrombotic events associated with COX-2 inhibitors such as rofecoxib and valdecoxib, resulting in their withdrawal from the market. 1. Celecoxib Celecoxib is a selective COX-2 inhibitor¾about 10-20 times more selective for COX-2 than for COX-1

Celecoxib is as effective as other NSAIDs in the treatment of rheumatoid arthritis and osteoarthritis,and in trials it has caused fewer endoscopic ulcers than most other NSAIDs.Probably because it is a sulfonamide,celecoxib may cause rashes.It does not affect platelet aggregation at usual doses.It interacts occasionally with warfarin%as would be expected of a drug metabolized via CYP2C9. Although celecoxib is associated with about half the gastrointestinal side effects of nonselective NSAIDs,the frequency of other adverse effects approximates that of other NSAIDs.Celecoxib causes no more edema or renal effects than other members of the NSAID group,but edema and hypertension have been documented. 2.Etoricoxib Etoricoxib,a bipyridine derivative,is a second-generation COX-2-selective inhibitor with the highest selectivity ratio of any coxib for inhibition of COX-2 relative to COX-1.It is extensively metabolized by hepatic P450 enzymes followed by renal excretion and has an elimination half-life of 22 hours.Etoricoxib is approved in the United Kingdom for the treatment of the signs and symptoms of osteoarthritis(60 mg once daily)and rheumatoid arthritis(90 mg once daily),for treatment of acute gouty arthritis (120 mg once daily),and for relief of acute musculoskeletal pain(60 mg once daily).Approval in the USA is still pending.Ninety mg daily of etoricoxib has superior efficacy compared with 500 mg of naproxen twice daily in the treatment of rheumatoid arthritis over 12 weeks.Etoricoxib has similar efficacy to traditional NSAIDs for osteoarthritis,acute gouty arthritis,and primary dysmenorrhea and has a gastrointestinal safety profile similar to that of other coxibs.Since etoricoxib has structural similarities to diclofenac,it is appropriate to monitor hepatic function carefully in patients using this drug. 3.Meloxicam Meloxicam is an enolcarboxamide related to piroxicam that has been shown to preferentially inhibit COX-2 over COX-1,particularly at its lowest therapeutic dose of 7.5 mg/d.It is not as selective as the other coxibs and may be considered "preferentially"selective rather than "highly"selective.The drug is popular in Europe and many other countries for most rheumatic diseases and has recently been approved for treatment of osteoarthritis in the USA.Its efficacy in this condition and rheumatoid arthritis is comparable to that of other NSAIDs.It is associated with fewer clinical gastrointestinal symptoms and complications than piroxicam,diclofenac,and naproxen.Similarly,while meloxicam is known to inhibit synthesis of thromboxane A2,it appears that even at supratherapeutic doses its blockade of thromboxane A2 does not reach levels that result in decreased in vivo platelet function.Other toxicities are similar to those of other NSAIDs
Celecoxib is as effective as other NSAIDs in the treatment of rheumatoid arthritis and osteoarthritis, and in trials it has caused fewer endoscopic ulcers than most other NSAIDs. Probably because it is a sulfonamide, celecoxib may cause rashes. It does not affect platelet aggregation at usual doses. It interacts occasionally with warfarin¾as would be expected of a drug metabolized via CYP2C9. Although celecoxib is associated with about half the gastrointestinal side effects of nonselective NSAIDs, the frequency of other adverse effects approximates that of other NSAIDs. Celecoxib causes no more edema or renal effects than other members of the NSAID group, but edema and hypertension have been documented. 2. Etoricoxib Etoricoxib, a bipyridine derivative, is a second-generation COX-2-selective inhibitor with the highest selectivity ratio of any coxib for inhibition of COX-2 relative to COX-1. It is extensively metabolized by hepatic P450 enzymes followed by renal excretion and has an elimination half-life of 22 hours. Etoricoxib is approved in the United Kingdom for the treatment of the signs and symptoms of osteoarthritis (60 mg once daily) and rheumatoid arthritis (90 mg once daily), for treatment of acute gouty arthritis (120 mg once daily), and for relief of acute musculoskeletal pain (60 mg once daily). Approval in the USA is still pending. Ninety mg daily of etoricoxib has superior efficacy compared with 500 mg of naproxen twice daily in the treatment of rheumatoid arthritis over 12 weeks. Etoricoxib has similar efficacy to traditional NSAIDs for osteoarthritis, acute gouty arthritis, and primary dysmenorrhea and has a gastrointestinal safety profile similar to that of other coxibs. Since etoricoxib has structural similarities to diclofenac, it is appropriate to monitor hepatic function carefully in patients using this drug. 3. Meloxicam Meloxicam is an enolcarboxamide related to piroxicam that has been shown to preferentially inhibit COX-2 over COX-1, particularly at its lowest therapeutic dose of 7.5 mg/d. It is not as selective as the other coxibs and may be considered "preferentially" selective rather than "highly" selective. The drug is popular in Europe and many other countries for most rheumatic diseases and has recently been approved for treatment of osteoarthritis in the USA. Its efficacy in this condition and rheumatoid arthritis is comparable to that of other NSAIDs. It is associated with fewer clinical gastrointestinal symptoms and complications than piroxicam, diclofenac, and naproxen. Similarly, while meloxicam is known to inhibit synthesis of thromboxane A2, it appears that even at supratherapeutic doses its blockade of thromboxane A2 does not reach levels that result in decreased in vivo platelet function. Other toxicities are similar to those of other NSAIDs