
01-Antibody
01-Antibody

Antibody From Wikipedia,the free encyclopedia An antibody(Ab).also known as an immunoglobulin(Ig).is a large,Y-shaped protein produced mainly by plasma cells that is Antigens the pathogen,called an ar region.Each tip of the"Y"of an antibody contains a paratope (analogous to a lock)that is specific for one particular epitope (similarly anal ogous to a key)on an antigen. allow ing these two gen-bind ing s el for attack by other parts of the immune system,or can neutralize its target directly (for example,by blocking a part of a microbe that is for its invasi ial).Depending on antigen, the foreign substance.The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region(located at the e of th on site h )which a con 0nn尚n bodies9 Antibo小y mmune syste ntibodies re secrete adaptive toa lock and key immune system, E sty by sical fo that is from the celtobe freen the.and a membrane-bound form that is attached to the surface of a B cell and is referred to as the B-cell ceptor(BCR).The BCR is found only on the surface of B cells and to either antibody fact ,61 of the B cel to produce full activation of the B cell and,therefore,antibody generation follow ing an n binding.Soluble antibodies are released into the blood and tissue fluids,as well as many secretions to continue to survey for invading microorganisms. Antibodies are gly coproteins belonging to the immunoglobulin superfamily4]They constitute most of the amma globulin fraction of the blood proteins.They are typically made of basic structural units-each with two large heavy chains and two small light chains.There are several different types of antibody heavy chains that def e five different types of fragments(Fc)that may be a ach d to the antige inding able to hind to its ep which is essentially the BCR).thus alowng the complex to mediate different role dependingon he ycn)preet at comerdThe ftbdibind The ability of antibodies to bind to ect th or eacn c rent type of ey histamine release a's Fab parat binds to all raic antigon for 黑高Re欧gan0 Though the general structure of all antibodies is very similar.a small region at the tip of the protein is extremely variable,allowing millions of antibodies with slightly different tip structures,or antigen-binding sites,to exist.This region is known as the hypervariable region.Each of these variants can bind to a different

antigen.2]This enormous diversity of antibody parato on the antigen-binding fragments allows the immune system to recognize an equally wide variety of antigens.The large and diverse population of antibody paratope is generated by random recombination events of a set of gene segments that encode different antigen- mgs品9ps,6 ed byndomhothe8心.hcr binding sites (o nis recom national process that produces clonal antibody paratope diversity is call e of D,and one of J is chosen.These gene segments are then joined together using random genetic recombination to 一hen0peeL y that retains the antigen-spe Contents .4.1 Immunoglobulin domains .4.2 Heavy chain CDRs.FV.Fab and Fc regions .5 Function 5.3 Natural antibodies ·6 :品 .6.3 Somatic hypermutation and affinity maturation .6.6 Asymmetrical antibodies Os)

Forms The membrane e-bound form of an antibody may be called a surface im unoglobulin(slg)or a membrane B The BCR】 when a specifi duction.o Iicrometer in diameter,on lipd afs that the BCRs 一器 trom competing intluences Antibody-antigen interactions determinants Antibody and antigen interact by spatial complementarity (lock and key).The molecular forces involved in the h n is relativ e rather than absolute.Relatively binding also means it is possible for an antibody to cross-react with different antigens of different relative affinities. Often,once an antibody and antigen bind,they become an immune complex,which functions as a unitary object and can act as an antigen in its own right,being countered by other antibodi es.Similarly,haptens are ct is antigenic Isotypes mammals there are five manmh each heavy cha cally:a(alpha),Y(gamma),(delta),(epsilon),and u(mu).This The antibody isotype of a B cell changes during cell development and activation.Imm never been ex to an antigen,express only the IgM IgM and IgD.The co- B cell ready to respond to antigen.]B cell activation follows engagement of the cell-bound antibody molecule with a n antigen,causing the cell to divide and differentiate into an antibody-producing cell called a plasma cell. In th ted form,the B c rther than a the ion of ant IgG,that have defined roles in the immune system
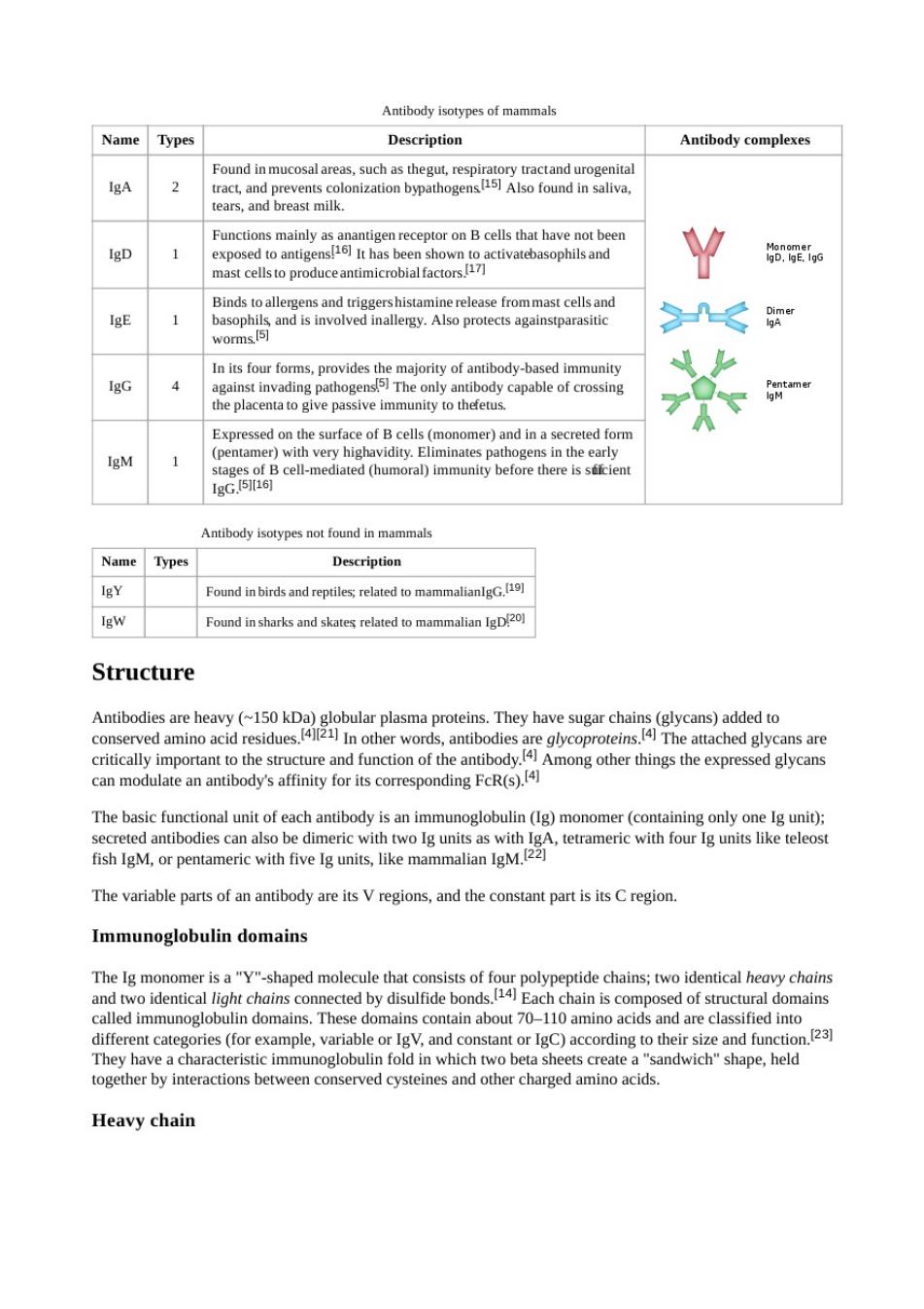
Antibody isotypes of mammals Name Types Description Antibody complexes 2 M exposed to antige ooaivabasophand .g antimicro Binds to allerg 1 gens and triggershistamine release frommast cellsand -≤ In is four forms,provides the mjority of antibody-based immuiy Expressed on the surface of B cells(monomer)and in a secreted form gM ges of Bumoral)immunity before there is srien Antibody isotypes not found in mammals Name Types Description IgY Found inbirds and reptiles;related to mammalian Igw Found insharks and skates related to mammalian ID Structure lobular che critically can modulate an antibody's affinity for its corresponding FcR(s). The basic functional unit of each antibody isan imm with unoglobulin(Ig)m with fish IgM,or pentameric with five Ig units,like mam malian IgM.(2 The variable parts of an antibody are its Vregions,and the constant part is its C region Immunoglobulin domains The monomer shped moleculehatsf opolypepide chainseichns Each chain is composed of structural domains n domains.The contain about 70 s and are clas d 23 fold in whichw a"sandwich"shape.held together by interactions between conserved cysteines and other charged amino acids. Heavy chain
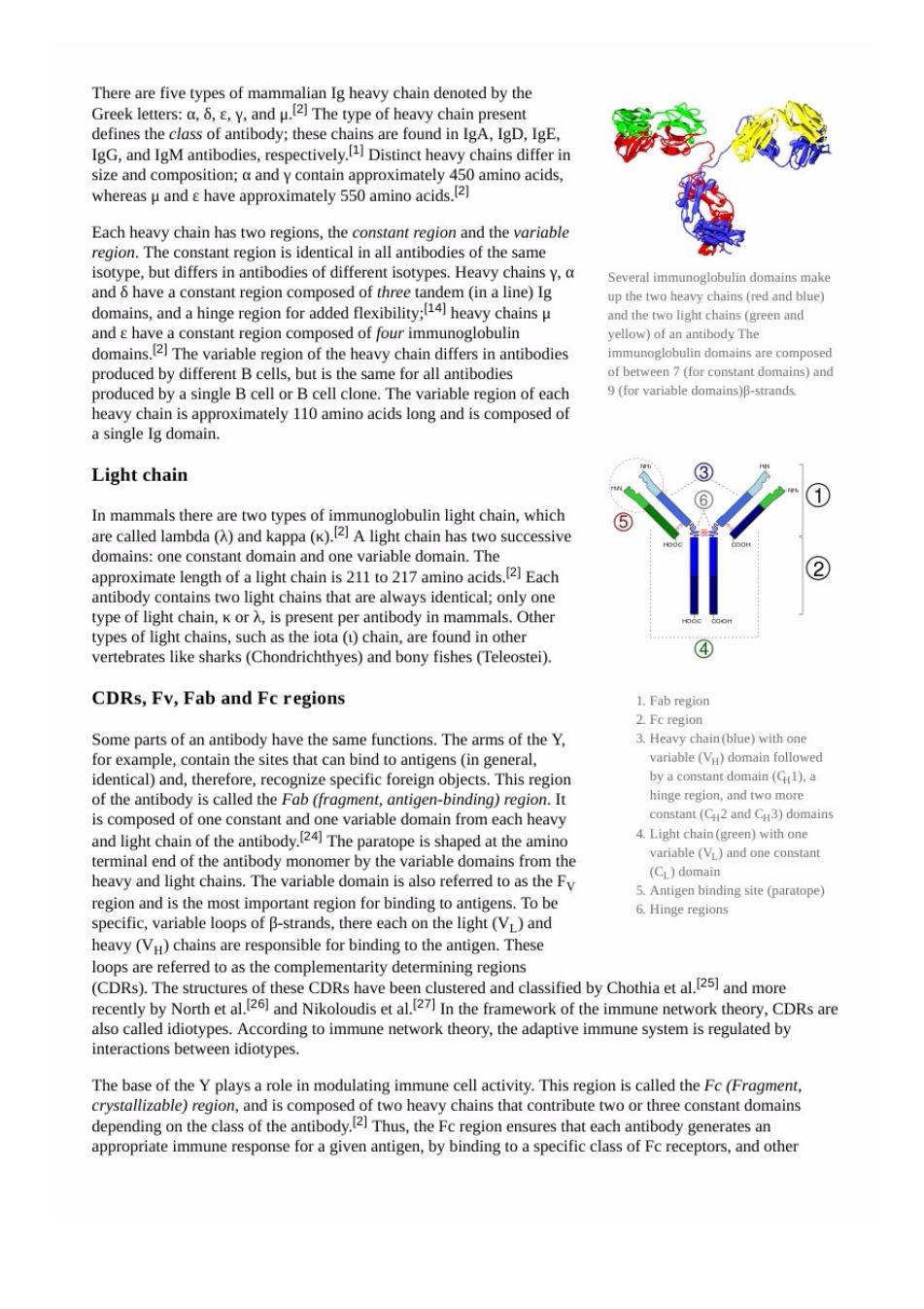
There are five types of mammalian Ig heavy chain denoted by the Greek letters:a.8..Y.and u.(2]The type of heavy chain p sent defines the class of antibody:these chains are found inA,ID,IgE. IgG,and IgM antibodies,respectively Distinct heavy chains differ in Each heavy chain has two regions,the constant region and the variable region. stantregon I in all antibo of the same and 6 ha Several domains mak domains.and a hinge region for added flexibility:heavy chains p the two口 and have a constant region composed of four immunoglobulin ellow)of an domains The variable region of the heavy chain differs in antibodies cell clo or al 9(for variable domains)-strand a single Ig domain. Light chain ① in mammals there s two successive domains:one constant domain and one variable domain.The approximate lengh of a light chain acids.Each antibod s two light chains that are always identical;only one type on Ch K or vertebrates like sharks (Chondrichthyes)and bony fishes (Teleostei). ④ CDRs,Fv,Fab and Fc regions 1.Fab region Some parts of an have the s.The arms of the Y. Fc region the sit variable V doman followed identical)and,therefore,recognize specific foreign objects.This region byaconstan domain(G).a of the antibody is called the Fab (fragment,antigen-binding)region.It and light 4.Light chain (green)with one chain of【he ody. riable (VL)and one constant and is the most in aion for hinding To be binding site(paratope) specific.variable loops of B-strands.there each on the light (and 6.Hinge regions heavy(Vu)chains are responsible for binding to the antigen.These loops are referred to as the complementarity determining regions couy Nor and的Ch mon In the framework of the immune network theory.CDRs are otype 8 ol he pay水hiis@ able compost 2)Th 0 s that con bute two or constant do nain

immune molecules.such as complement proteins.by doing this.it mediates different physiological effects aam时n l In summary.the fab region of the antibody determines antigen specificity while the fc region of the antibody determines the antibody's class effect.Since only the constant domains of the heavy chains make up the Fc region o fects.Possible classes of e alpha. ntib nd M of antibodies include:Opsonisation,agglutination,haemolysis,complement activation,mast cell degranulation and neutralisation (though this class e ffect may be mediated by the Fa st Fc mediated effects are directed a Function The main categories of antibody action include the following ggutination,in which antibodies"lue together"foreign cells into clumps that are attractive targets for phagocytosis e or ph soluble antigens,forcing them to precipitate ou tion (fix ation),in which an ntibodie that are vitn a membrane complex,w hiletoinfoiegnoalencourage i .Encouragement of inflammation by chemotactically attracting inflammatory cells Activated b cells differentiate into either antibody roducing cells called plasma cells that secrete soluble antibody or memory cells that survive in the body for years afte rememerded inde to allow the immun system to vided by oassive immunization from lating antibodies are prod by clor al B ce protein ute to i ty in t macrophages and other cells by coating the pathogen:and the trigger destruction of pathogens bysmothermmune rponsuhas the compeme paybesoier oactive amine degranulation to contributetoimmunity against certain types of antigens(helminths, Activation of complement res ling of bacteria in two ways. First,the binding of the antibo dy and complement molecule a pro 559 op nese pnag components for embrae tack complex tt antibodie to kl the bacerdi

Activation of effector cells To combat pathogens that replicate outside cells,antibodies bind to pathogens to link them together,causing them to agglutinate.Since an 2 antibody has at least two paratopes,it can bind more than one antigen l epitopes cam oe sur es of these antigen ogens have Fo receptor on a particular cell triggers an effector function of that cell; eutrophils will microbe The activation of natural killer cells by antibodies initiates a cytotoxic mechanism known as antibody-dependent cell-mediated cytotoxicity (ADCC)-this process may explain the effica oclonal antibodies us d in bio system approp Natural antibodies Humansand higher"that re viral en d the classica complement pathway leading to lysis of enveloped virus particles ong before the adaptive immune response is activated.Many natural antibodies are directed against the disaccharide galactose (1,3)-galactose(a-Gal), which is found satemitnlsgaronghcovlhlodcelsurfaoeP9lesandgCneatedinponseo s sugar by bac ting in the serumo antigen Immunoglobulin diversity an antibody resp of mic aries allowin them to interact with many different antigens 34 It has been estimated that humans generate about 10 billion different antibodies,each capable of binding a distinct epitope of an antigen.Although a huge repertoire of different antibodies is generated in a single individual,the number of genes available to make th ese proteins is limited by the size of the humar genome entaieBoestogmerateadesepeol antibody genes.36 Domain variability The chromosomal region that encodes an antibody is large and contains several distinct gene loci for each domain of the ound on )are 22 and 2 in hur of these domai hich nt in heavy and light chain of every antibody,but can differ in different antibodies generated from distinct B cells (C)D

within the variable domains by conserved framework regions.Tthe heavy chain locus contains about 65 different variable domain genes that all differ in their CDRs.Combining these genes with an V(D)J recombination the heavy chain are shown in red PDB:1GT) immunoglobulin heavy or light chain is encoded in several pieces be(V (e variable (V).div ersity(D)and joining ()segments. aa084H54H0 6行 segments exist and are tandemly arranged in the genomes of ③: mammals.In the bone marrow,each developing B cell will 70 as emble ar region by randomly a国÷1 copies of each tvpe of and different of gene segments can be used to generate each immunoglobulin va region s pro ss ger erates a huge num t antiger al ombinatio (i.e.V2 family)for lambda light chain immunoglobulin is coupled of-9. with the activation of microF ional imn onyone kind of variable chain. Somatic hypermutation and affinity maturation pgactvaionwihaniger Follow B cells begint o pro rate rapidly. n these rapidly dividing cel s,the genes called somr mutation (SHM).SHM sults in approximatelyone eotide cha er celdivisionsa consequence.any daughter Bcells will acquire slight amino acdd variable domains of their antibody chains. This ser ngerinr h arfinity)cls thateir dies on their surface will receive d wi erea affinity not ntibodies to
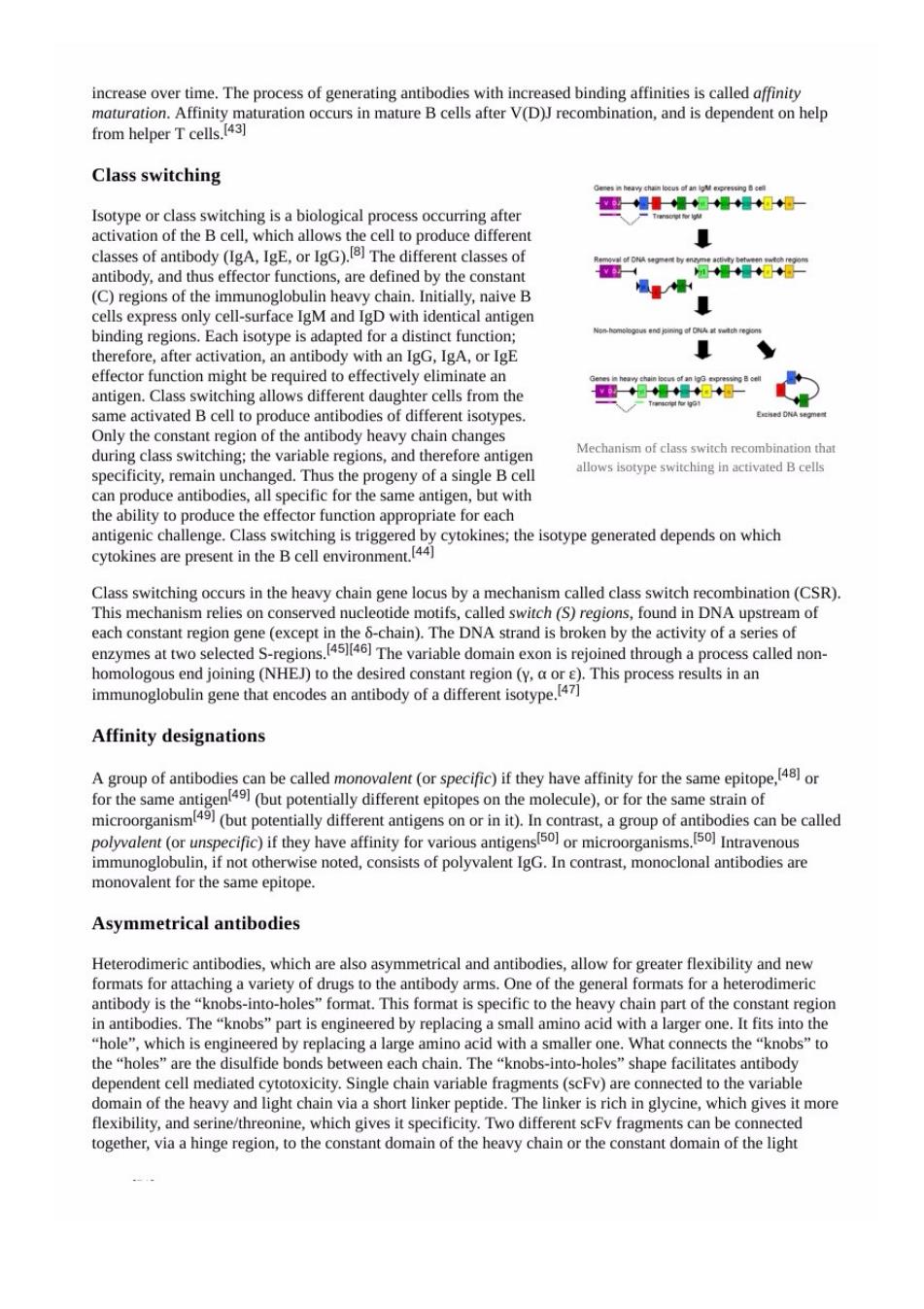
nerating antibodies with in ased binding affinities is called affinity Class switching or class switching is a biological p o afte classes of antibody(IgA,IgE,or IgG).The different classes of antibody,and thus effector functions,are defined by the constant ( ,家 he immunoglob eB binding regions.Each isotype is adapte therefore,after activation,an antibody with an IgG.IgA,or IgE effector function might be required to effectively eliminate an antigen.Cl 国g“ Only the constant region of the antibody heavy chain ch during class switching:the variable regions,and therefore antiger Mechanism of cla witch r tha specificity.rema in unchanged.Thus the progeny of a single Bcell n produce ant he same antgen.bu antigenic challenge.Class switching cytokines are present in the B cell environment.44 mre in tho he chain gene locus by a mechanism called class switch re mbination(CSR) This mechanism relies on conserved nucleotide motifs,called switch (S)regions,found in DNA ups am of ech t n (an)Thed isoken byhe city ofof enzymes at two selected s The variable domain exon is rejoined through a process called non- EJ)to the desir Affinity designations A group of antibodies can be called monovalent(or specific)if they have affinity for the same epitopeor 一 microorganism mentor unspecific)if they have amfnity or microorganisms. Intravenous e noted,consists of polyvalent IgG.In contrast,monoclonal antibodies are mo me epitope Asymmetrical antibodies which are also asymmetrical and antibodie allow for greater flexibility and new of th in antibodies The "knobs"nart is engineered by replacing a smallamino acid with a larger one.It fits into the "hole",which is engineered by replacing a large amino acid with a smaller one.What connects the"knobs"to the "holes"are the d ulfide bonds between e chain.The "knobs-into-holes shape facilitate es antibody d tide.The necte s rich i oe it r ,and serine/which gives it specificity.Two different scFfragments can be comected more together,via a hinge region,to the constant domain of the heavy chain or the constant domain of the light