
03-Monoclonal antibody therapy Monoclonal antibody therapy clonal antibodies (mAb) tively apyandioactiedbselocal 之c es on a Contents :2 body structure and function … 4.3A rug conjuga ne therapy Antibody structure and function mately 150 kDa and are co ed of two kinds of Da)and the lig o(n da ()By for the Some some m s include ErbB2. and are m%of bres cancer tmor celSuch breow HERposiive specific antigens presented on the surfaces of tumors. History on n und mAb lly short-liv rith blood
03-Monoclonal antibody therapy

Murine gufx b).These na technolo for which an MA点. Fv,single-chain Fv fragm gy.tran Chimeric and humanized To reduce murine antibody imm enicity (attacks by the in ainst the antibody),murine molecules were en ered to ent a une syTh was in ly a d by the prod at murin Human monoclonal antibodies Targeted conditions Cancer Autoimmune diseases Monoclonal antibodie mab.which are effective io rhe is by their abil ofadou (E)and is moderate-severe Alzheimer's disease Therapy types Radioimmunotherapy

Radioim Antibody-directed enzyme prodrug therapy Antibody-drug conjugate Immunoliposome therapy Checkpoint therapy the TME sed by B cells FDA approved therapeutic antibodies

Brand name Company e n 1222194 chimerc Fab 1a357 Abbvie 12312002 fully haman TNE 125057 A.mgen 9232016 TNF 61024 2222m1n HER2 25 57/21 CD52 242015 ully huma 2555 5/182016 o 6103 101B2016 6104 EMD Sereo 3232017 PD-LI 7610 5/12/1930 chimeric 1037 39201 fully haman Avastin 2262004 humanized VEGF 12500 Merck 10212016 1232014 125557 gea 12530 215201 761032 6/172009 125319 10281996 6 UCB(con 4222008 TNF Croho's disease 12516间 2/120m EGER Roche 12101997 且2RA 11/162015 fully heman
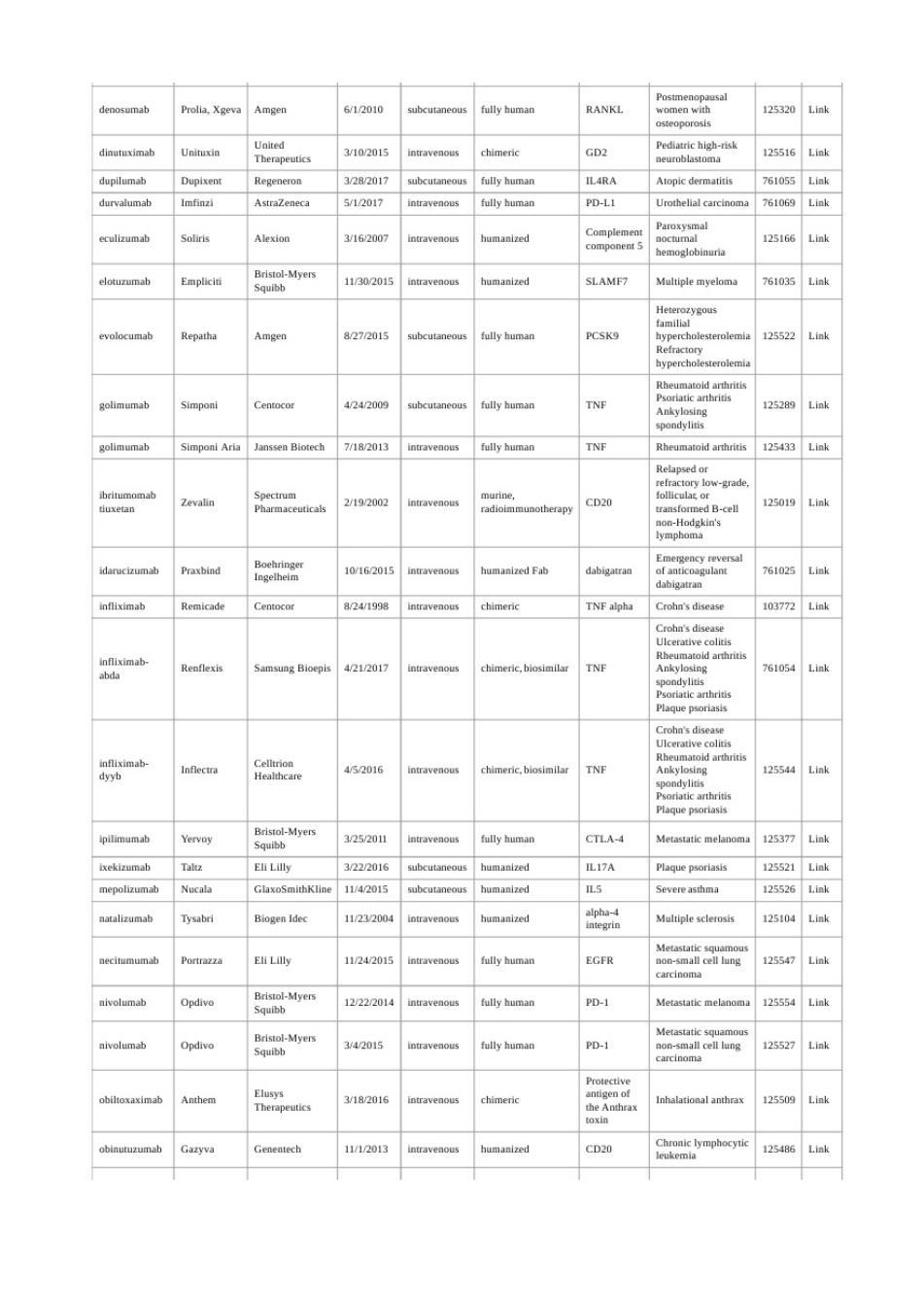
Prolia,Xgeva Amge 612010 ully huma RANKI 125120 /10201 GD 12551 3282017 fully human 761055 5/12017 fully heman PD-LI 61069 3y162007 2516 113020151 humanized 76103 8272015 fully haman PCSK9 1255 4242009 fully hama 2528 7/12013 TNF 125433 219202 2501 6 10y162015 6102 421201 TNE 452D16 chimeric,biosimilar TNF Bris-Myers 32520 CTLA-4 Eli Lilly 3222016 IL17A 1142015 ILS 232D04 1242015 EGER 122220144 PD- 12555 6 pdivo 342D15 PD-1 182016 halational anthra 12550 12540
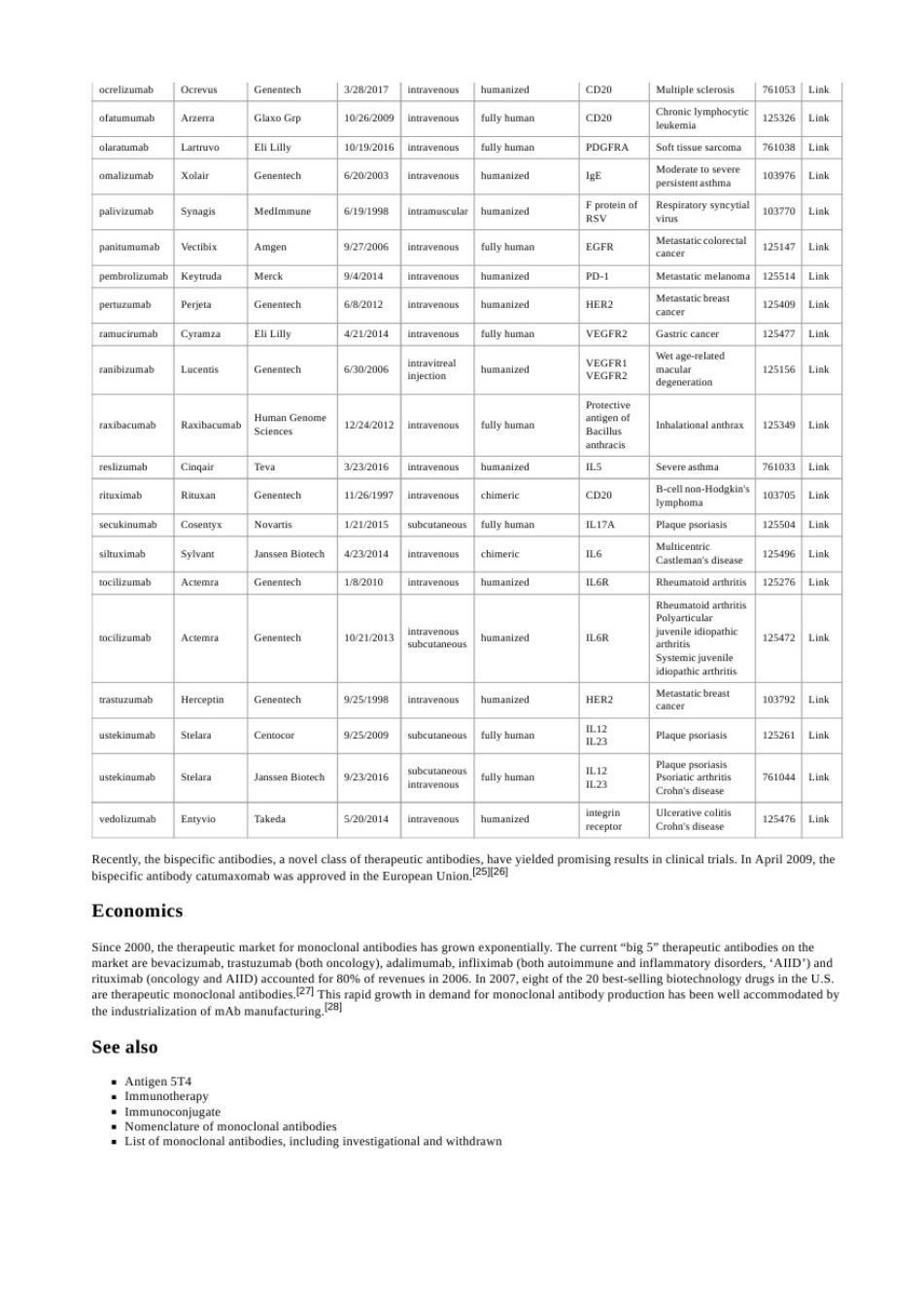
Genentech 322017 CD20 Multiple sclerosis 761063 02620 m 10y192016 fully homan PDGFRA 761038 Genentech 620203 IgE 1097 6/19/1998 10377 927206 fully human EGFR 12514n Merck 942D14 PD-1 682012 HER2 EL LUly 4212014 tul hama VEGFR2 125477 6302006 VECPR 12515 2242012 fully hama 12534 Teva 3232016 Severe asthma 76103 126197 Hodgkin 1/21/2015 subcv fully heman IL17A 125504 Janssea Biotech 4232014 chimeric 12549 1a2010 6 12527 0212013 Herceptia tech 925/1938 HER2 10379 9252009 fully homan 12526 anssen Biotech 9232016 fully human Ctoba's disease mab 5s202014naw humanized 123476 ults in clinical trials.In April 2009.the Economics Since 2000.the the ally The h mab (both o and n20 of the 20 2 rapid grow See also .Antigen 5T4

References 1.Wal E.Hume SD.Jo hp: 10.10732Fp121370 c():(h CA I rland science.ISBN 0-443-07310-4. 405-0 MD7427932 n nih.go ed7427932 es in the treatm of leukemia and lymphomaBlood.59 PMID 7 d703262 54(1 PMID I .ncbi.nlm.nih. /10.1016% 9-34.PMID 12514726(https://www.ncbi.nl 472 of an anti-pHaboy for WL.R M.Kon 89(10) 89.4285C( 877-3. 42877a E MPU %2Fmabs.1. Baldrick P.Bu se o )20001( primat 1(5 16PMC259 1 dyA,SinghRa 2016.Ev H P 521665549 L0g10.117 1BN0-443-07145-4 m the alz ch /s1 21:/pubmed/21784348) ma Sk.Sp en A).Hope E. er. ao th (https://www

10.32 23 204 (https://de 11 do. D1902291p dies to MC. )0( 4161D g10.4161 28 the bi MC27 416 944 External links Cancer Management Handbook:Principles of Oncologic Pharmacotherapy (registration reguired Retrieved from "https://en.wikipedia.org/w/index.php?title=Monoclonal antibody therapy&oldid=799029451 ·T 2017.t0621