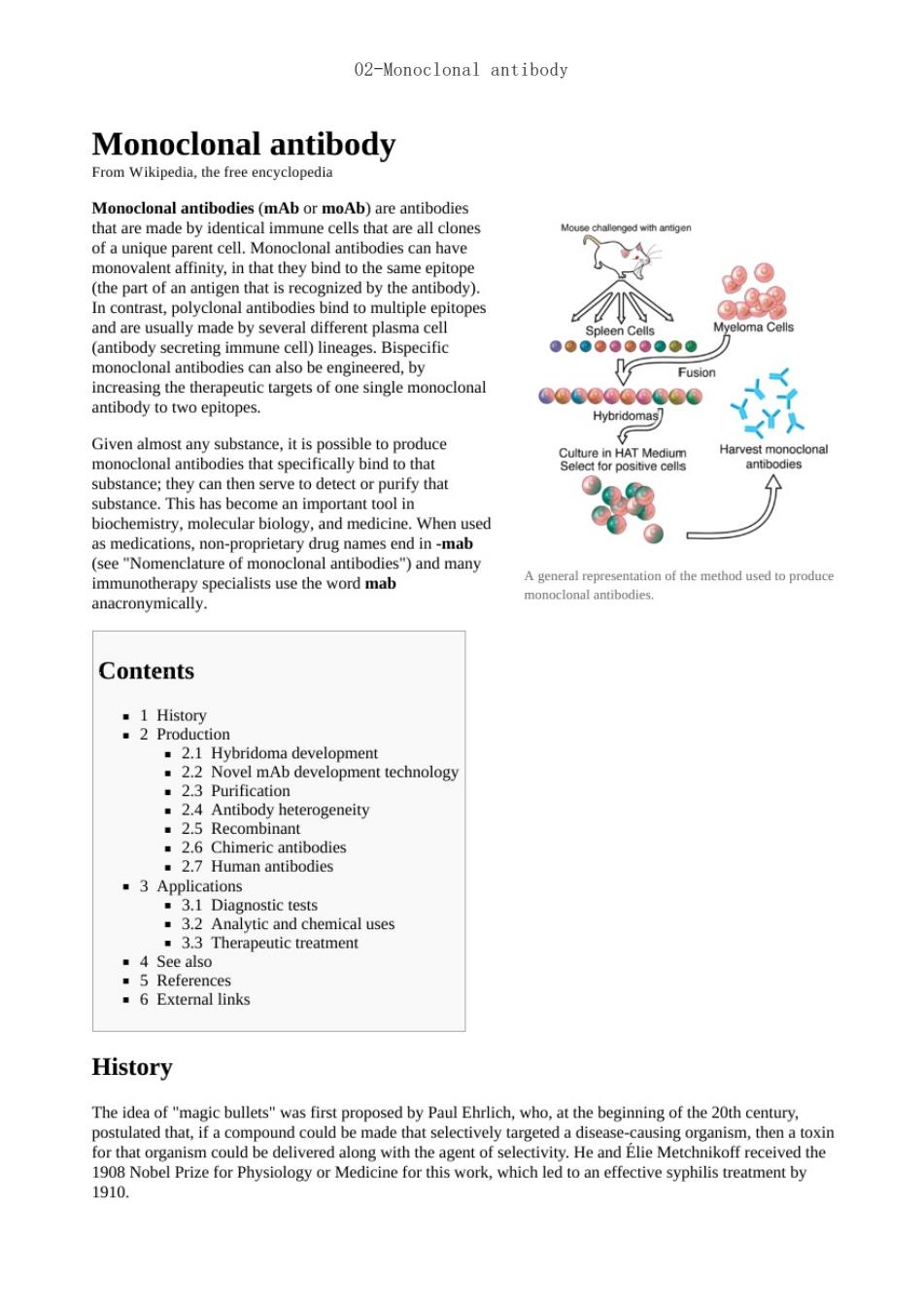
02-Monoclonal antibody Monoclonal antibody From Wikipedia,the free encyclopedia Ab)are antibodies e ma monovalent affinity,in that they bind to the same epit (the part of an antigen that is recognized by the antibody). es. monoclonal antibodies can also be engineered,by increasing the therapeutic targets of one single monoclonal antibody to two epitopes. Given almost any substance,it is possible to produce antibodies that specifically bind to tha biochemistry,molecular biology,and medicine.When used as medications,non-proprietary drug names end in-mab (see "Nomenclature of s")and many immunotherapy specialists use the word mal A general of the method used to produce Contents Hybridoma developmen 2.3 Purification development technology .2.4 Antibody heterogeneity .2.7 Human antibodies 3.3 Therapeutic treatment : .6 External links History The idea of"magic bullets"was first proposed by Paul Ehrlich,who.at the beginning of the 20th century postulated that,if a compound could be made that selectively targeted a disease-causing organism,then a toxin hnikoff received the 1910. to an effective syphilis treatment by
02-Monoclonal antibody

In the 1970s.the b-cell cancer multiple mveloma was known.It was understood that these cancerous b-cells all 股ma8的e In 1975.Georges kohler and cesar milstein succeeded in making fusions of mveloma cell lines with b cells to create hybridomas that could produce antibodies,specific to known antigens and that were immortalized.2] They shared the Nobel Prize in Physiology or Medicine in 1984 for the discovery.2] Production Hybridoma development monoclonal antibodies are typically made by cell culture that involves fusing myeloma cells with mouse spleen cells immunized with the desired antigen.Rabbit B-cells can be used to form a rabbit hybridoma Polyethyl lisused to fus s,but the rwis used This is possible because mveloma cells have lost the ability to synthesize hypoxanthine-guanine-phosphoribosyl transferase ng at slides o (HGPRT),an enzyme necessary for the salvage synthesis of nucleic acids altures of cells that mak not a problem se cells ess the de nov hese an a folic acid anal nalying the pro cts to select the most promising of them makes them unable to use the de novo pathway and become fully auxotrophic for nucleic acids,thus requiring supplementation to survive The selective culture medium is called HAT medium because it contains hypoxanthine,aminopterin and thymidine.This medium is selective for ma)cells.Unfused myelom of their limit hbrid cell.referred to as hybridomas,are able to grow indefinitely in the media because the spleen cell partner supplies HGPRT and the myeloma partner has traits that make it immortal (similar to a cancer cell). unlimited quantities in the bottle This mixture of cells is then diluted and clones are grown from single hown in this picture parent cells on microtitre wells.The antibodies secreted by the different clones are sayed for ost productive and stable clone is ther The hybridomas y. indefinitely ina suitable cel cu edium.They can also be inj ng the gut ney pro called ascites fluid. The medium must be enriched dur selection to further favour hybrido wth This aaemoa Novel mAb development technology

Several monoclonal antibody technologies had been developed recently such as phage display,single Bcell culture single cell amplification from various B cell populationsand single plasma cell interrogation technologies.Different from traditional hybridoma nologies use mol nalian s technology.One of the advantages of the new technologies is applicable to multiple animals,such as rabbit,llama,chicken and other common echnician hand-filling wells with iquid for a re ch test.This tes experimental animal hybrpanmtionofe Purification ties to produce desire ody.This is etected by fusing After obtaining either a media sample of cultured hybridomas or a sample of ascites fluid,the desired antibodies must be extracted.Cell cuture sucn as likely to have host antibodies.pr SaDcleehangmpe5 viruses.In both cases,other secretions by the hybridomas such as cytokine s may be present.There may also be bacterial contamination and. as a resu he bacte ending on th one or the other method (in vivo or in vitro)may be preferable ple is first centrifugation followed by filtration with a0.45 m filter.These large es slides of m particles can cause a phenome non called membrane fouling in later on st s.In addi he of product in the sample by ultrafiltration or dialysis Most of the charged impurities ally anions such as nucleic acids and endotoxins These can be separated by ion exchange chron aphy Either cation exchange chromatography is used at a low enough pH that the desired antibody binds to the column while anions flow through,or anion exchange chromatography is used at a high enough pH that the desired antibody flows through the colum n while anions ind to it.Various can a ng w the ctric point (p Ins,th rotein has a ne sitive cha of 4.8.which is significantly lower than that of most monoclonal antibodies,which have a pl of 6.1.Thus,at a pH between 4.8 and 6.1,the average charge of albumin molecules is likely to be more negative,while mAbs ged and he is possible to separate ther 0potamt,otcoter nd,has necessary for a goo Transferrin can instead oved by s atography. This method is more reliabl consistent and varyith Has molecule are proonated and deprotonated,while size stays relativelyconstant Nonetheless,it has drawbacks such as low resolution,low capacity and low elution times. a much quicker.single-step method of separation is protein ag affinity chromato selectively binds to protein A/G,so a high level of purity (generally>%)is obtained.However,this method

ph can cause protein a/g itself to leak off the column and appear in the eluted sample.gentle elution buffer systems that employ high salt concentrations are available to avoid exposing sensitive antibodies to low pH. Cost is aso an important consideration with this method because immobilized protenA/G is a more expensive resin To achieve maximum purity in a single step,affinity purification can be performed,using the antigen to provide specificity for the antibo ody.In this method the antigen used to genera ach The antibody-containing media is then incubated with the immobilized antigen,either in batch or as the antibody is passed through a column,where it selectively binds and can be retained while impurities are ombtter orore tl,gufestheever Antibody heterogeneity These varants are typicallyrn varanB,oxidized amino acid side chains,as well as amin These seemingly minute structural nty and proce on as we as th product poten monoclonal antibodies includes ca re of the p get with protein a elutio acidification to inactivate potential mammalian viruses,followed by ion chromatography,first with anion beads and then with cation beads. Displacement chromatography has been used to identify and characterize these often unseen variants in re suitable for subsequnt preal evluation remens such asmal phaacoknic Knowledge gained during the preclinical development phase is critical for enhan ed produc ing and prov gement anc sand pro Recombinant display ast.rather than mice.These techniques rely on rapid cloning of immun lobulin gene segments to create libraries of antibodieshy different amino aid seqencefrom which antibodies specificities can be selected The phage antibody libraries are a variant of phage antigen librarie techniques can be use to e with which an Chimeric antibodies While structurally similar,differences between mouse and human antibodies were suffic ent to voke ar and the ent-mot antibodies (HAMA)

Recombinant DNA has been explored since the late 1980s to increase residence times in one DNAencodngthebndingpomionofamonocionalantbodywasmCrgeiAwihhnahSg proach,mouse roducine Dheen. Human antibodies Ever since the discovery that monoclonal antibodies could be generated.scientists have targeted the creation of "fully"human products to reduce the side effects of humanised or chimeric antibodies.Two successful approaches have been identified:transgenic mice2 I and phage display. As of November 2016.thirteen of the nineteen "fully"human monoclonal antibody therapeutics on the market were derived from transgenic mice technology. Adopting organizations who market transgenic technology include: Meda Abgenix-which marketed the Xenomouse technology.Abgenix was acquired in April 2006 by .Regeneron's Veloclmmune) .Kymab-who market their Kymouset onal OmniM Ablexis.LLC- ouse platform ) Phage display c phae dis press variable antibody domains on filam ntous phage coat proteins(Phage lay an antibody libraries against diseased and non-diseased tis 19g be used maahrdgameaiomapaeonhlpesdSmvhndiae,hiammorydiscs In August 2006 the Pha tical Re companies hac ials Administration( by the d Dru Applications Diagnostic tests sue sections and Analytic and chemical uses Antibodies can also be used to purify their target compounds from mixtures,using the method of immunoprecipitation. Therapeutic treatment
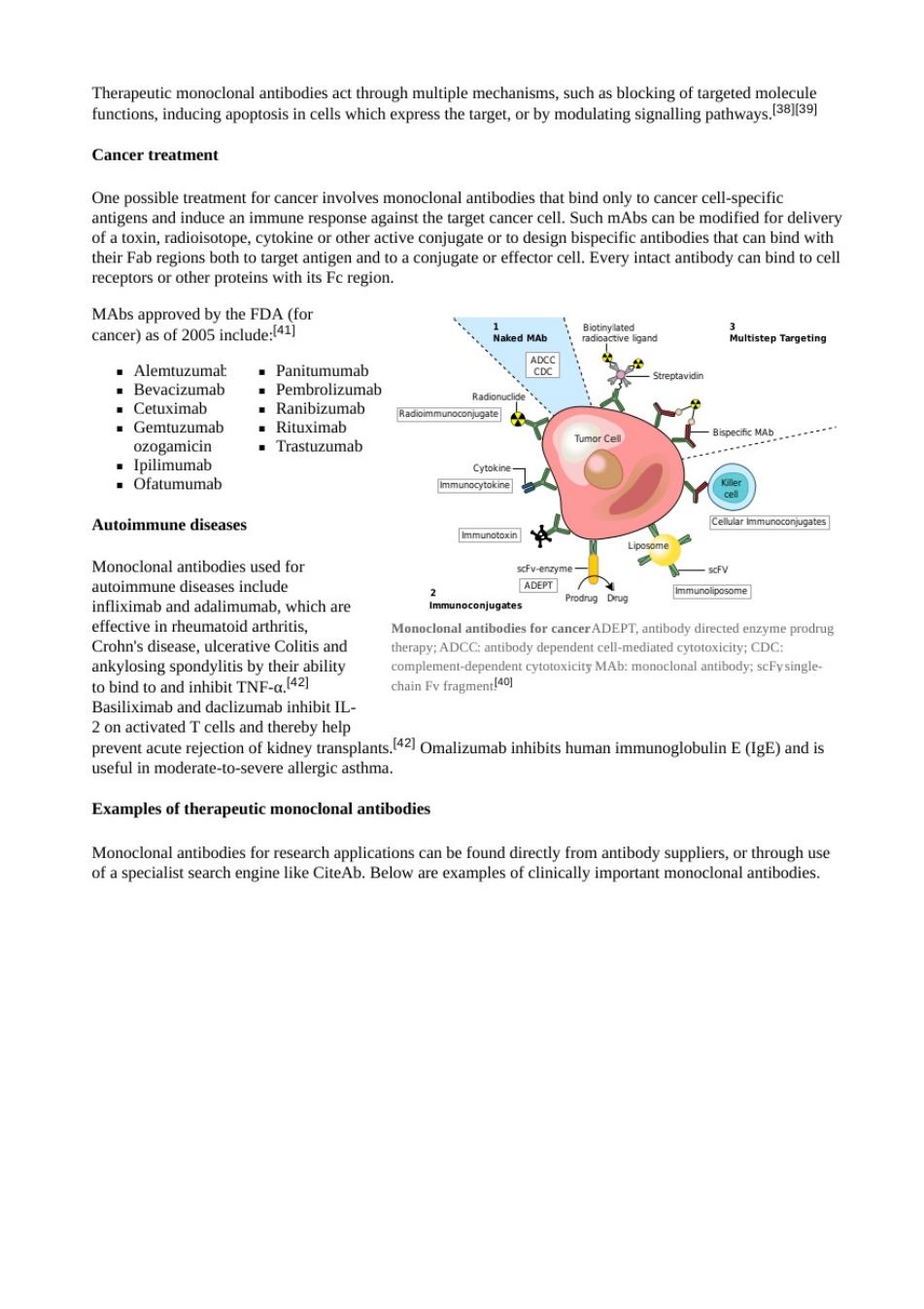
Cancer treatment for cancer involves mond of at other cdcanibodieshalcCntle their Fab regions both to target antigen and to a conjugate or effector cell.Every intact antibody can bind to cell receptors or other proteins with its Fc region. 公wm的RA Panitumumab 、 .Bevacizumab Pembrolizumab ozogamicin .Trastuzumab Autoimmune diseases Monoclonal antibodies used for A】 which are toid arthriti ncerADEPT antibody din Crohn's disease ulcerative colitis and herany ADCC antibody der ndent cell-mediatrd rytotoxicity:CDC ankylosing spondylitis by ther ability complemeat.depen nt cytotoxicity MAb:monoclonal antibody:scFysingle tobind to and inhibit TNF- chain Fy fragment T ction of kidney transplants.421 Omalizumab inhibits human immunoglobulin E(IgE)and is useful in moderate-to-severe allergic asthma. Examples of therapeutic monoclonal antibodies Monoclonal antibodie f a specialist search elow are examples of clin
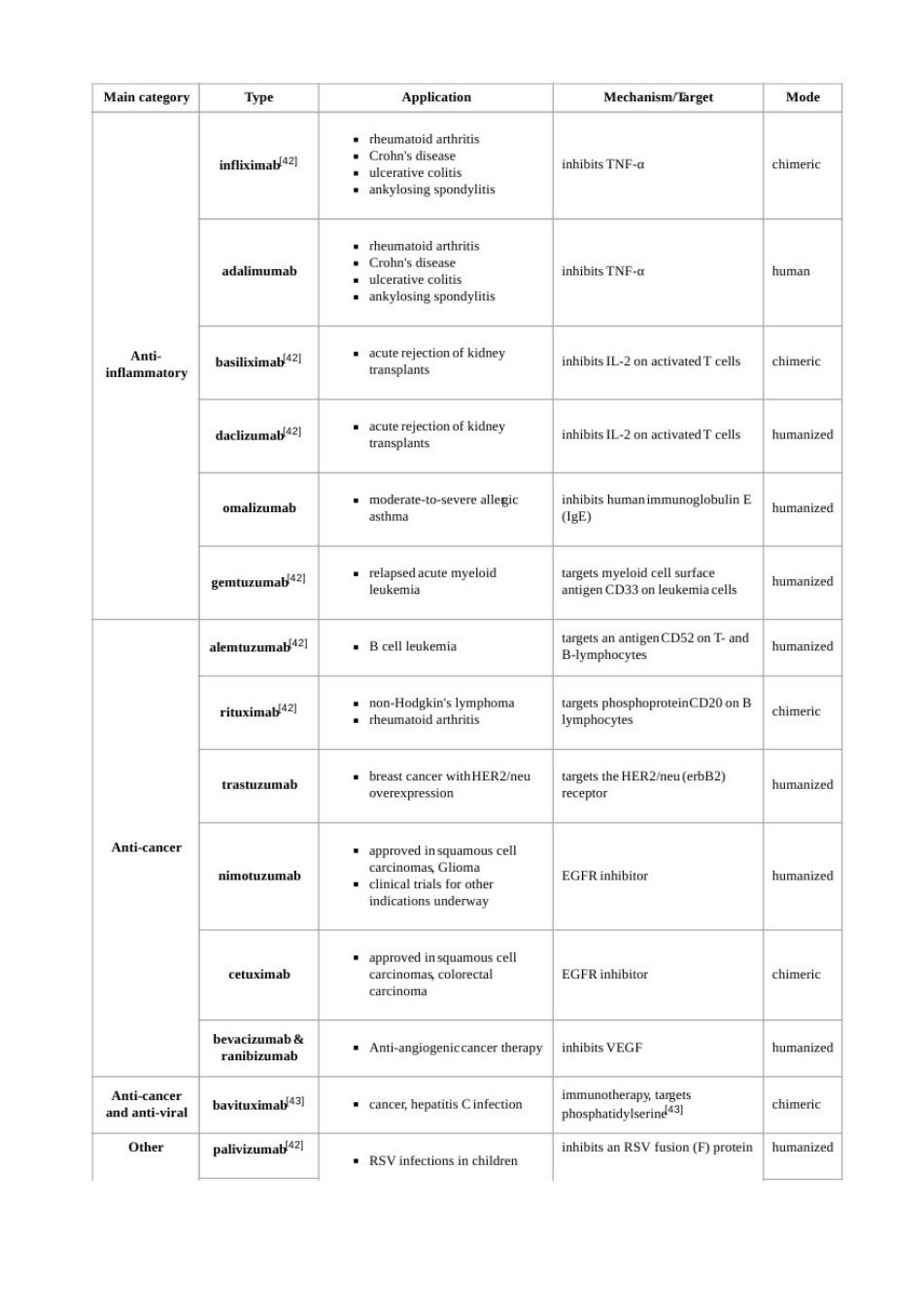
Main category Type Application Mechanism/Farget Mode ankylosing spondylitis .rheumatold arthritis adalimumab inhibitsTNF- human .ankylosing spondylitis inhibits atory daclizumab42 inhibits IL-2 on activatedT cells humanized omalizumab ·re eic humanized CDon ukemia cells humanized alemtuzumab42] 。Bcell leukemia n antigenCD52onT-and B-lyo humanized rituximab4☒ oteinCD20 on B chimeric trastuzumab ·2neu cHER2nau6b82 humanized Anti-cance us cell nimotuzumab EGFR inhibitor humanizec cetuximab EGFR inhibitor chimeric .Anti-angiogeniccancer therapy nhibits vege chimeric Other RSVinfections in children inhibitsn RSV fusion (F)protein humanized

See also Affimer Antibody mimetic :地 Immunotoxins,which sometimes use Polyclonal antibodies I antibodies References 2.Marks LV.A Healthcare Revolution in the Making:The Story of Cesar Milstein and Monoclonal Making monoclonal antibodies (hutp://www.whatisbiotechnology.org/exhbition/milsen/ M.Waldma H.Winter G()"Reshapinghum PMID 312 ang J1.Shen MH.Polv nediated coll fusi Methods Mol Biol.2006;325:59-66. 5.National Research C uncil (US)Committee on Methods of Producing Monoclonal ntibod NBk109BN978- 0-309-07511-4 Platform to Generate Functional Recombinant Monoclonal Antibodies Using Rabbit B Cells from .org/plosone/article?id=10.1371/journ Ipone.0086 LoS Ww. PMID 24503933(ht (htps://doi.org/10.1371%2Fjournal.pone.0086184). Schaefer,Anne:Young.James W Eric:Nussenweig. ce.301(56381374-1377.15SN1095-9203 (hutps: issn/1095.9203) cbmgov/pubmed/12920303)o10.126/cience.1006907(hup g.Qingzhao:Duty,Andrew;Helms,Christina;Mathias,Melissa pra.J.Donald (2007 6-01)."Mature B cells cl ass switched to N0021-038 1g79NCmh8器6尚oo2c7品saoi。 mc/articles/PMC1866247)

05.4 ovpmc/ariclesPMC)PMID8(https:/ww.ncbi.nim.nih.govpubmed/19 (hip://1 prot.2009.3). Qingzhao:Swiatkowski, Mike:Mathias,Melissa:Garman.Lor:Helms.Christina (2009-01-16)"Functional anergy ina P3SSSN1540-g38 ss5a0.9538.pC2626668htps d1910387 doi.org/ub Le 306 11 Turk.Ellen:Kang. 89).doi:10.1038/nprot..2013.117htps/doi.org/10.1038%2 Fnprot..2013.7 12 asak lonescu R (2008)."Hetergeneity of nsitive pubmed/19075686.dot10.2174138920108786786402 do.0r/10.21749%2F1389201 0.105:345-52.pMlD20414207 142Yhmd2o41427ot101038am277tps1oiomgn020207 D Ni variants in IgG1:Isolation,characterization,in vitro binding erties and pharmacokinetics in rats"(ht sbio cience.com/journals/mabs/abstract.php? 818176 cn6gNpbmd20e18176o416261333ps0oog91014161%m 2.6.13333) J.CanoT(A ink of iV0021-967311)00722-9). Chromato A.1218(315 86.PMID 21700290 (https://w w.ncbi.nl m/)doi-0.1016/j-chroma20105061(h:/)chroma 16.Rathore AS,Winkle H(January 2009)."Quality by design for biophar euticals".Nat.Biotechnol.27 (126 34PD19131992tps ww.ncbi.nlm.nih.gov/pubmed/19131992).doi:10.1038/nbt0109-26 17 caise de transfus ne.9(1):1 22 PMID 1889896 (hup 18."Dr.George Pieczenik"(http://www2.mrc-Imb.cam.ac.uk/component/content/article/40-archive/200-arch 1ve-servic alumni). Biology(LMB).17 2009. tion of antibodie S106-S12 PMID 10831134 (hutps:/www ncbi.nlm.nih.gov/pubmed/10831134).doi:10.1053/plac.1999.0511(https://doi.org/10.1053%2Fplac.199 20 Chadd HE.Chamow SM(A Biotechnol.12(2) 1128723 10.1016%2S095 9%8800%06d9287236).do:10.1016s0958-1669(0)y00198 21.Lonbe g N.Hus rD(9)."Human antibodies from transge ic mice".Int.Rev.Immunol.13(1):65- 710302030i0g0t87494109do1031008a301s50906i738 .org/1

22."Bristol-Myers Buys Medarex Drugmaker for $2.4 Billion(Update3)"(https://www.bloomberg.com/app 2a.AepdpaAc&ida2oVaXYGSgA 200062900 html platform.php) 27."Proprietary antibody platform"(hutp://ww rianni.com) c.net/p) tar dy plattorm"(htt Vw 、 ins".N ell Ds.com) r52M)nc.nm.nh.govpobm 30.MaK464 AD W rG19911."By mmunization.Joural of Molecular Biolog .222(3:581-97.PMD1748994p s://www.ncbi.nlm.n 48994).dot:10.1016/0022-2836(91)90498-U(htps:/do1.0rg/10.1016%2F0022-2836% 31.Carmen S.Jermutus L (2002)."Conce pts in antibody phage display".Briefings in Functional Genomics 101093/bfe 03.PMID 152 904(ht Im.nih.gov/pubmed/15239904). K "Methods Mol Biol.178:201-5 849ttpsncnLt82 bmed11968489). Abeloff.MD:Armitag 201 33. inesspark.co.uk/cay92125.htm) situ isolation of novel antibodies"(http:// D953 0562(hPs ncbi h.gov/pubme 95305 The Current State of Pro 0 chidh co ue3 1 ASp lenat/ icde/PIs0140.673600)01034-5 /fulltext.7 The Lancet..3559205:73540.PMD10703815hps 815).dot10.1016/s0140-6736(00)01034-5(htps:/do1.0rg/10.1016% 39.A stralian Prescriber (2006)."Monoclonal antibody therapy for non-malignant disease"(http://www.aust 40 -0 doi:10.1038/35101072htps:/do1.org/10.1038%2F35101072). 41 moto E. iry 01, Principles Pharmacotherapy(http://www.canc (Ed)Cancer Management (.com/cancer-management-1) 42.R (2003).Pharmacology.Edinburgh:Churchill Li nd mechanism and mode.ISBN 0-443-07145-4. 43Stansight.Bavituximab profile (hitp-/adisinsight.springer.com/drugs/800022616)Last updated External links .Monoclonal Antibodies,from John W.Kimball's online biology textbook