
上游充通大¥ e SHANGHAI JIAO TONG UNIVERSITY Chapter 9.Phase Diagram Il Immiscibility:不溶性 Spinodal Points:拐点 Simple Eutectic Diagram:简单共晶相图 Peritectic Phase diagram:包晶相图 Compounds:化合物 Conclusion Remarks Examples ONG UNN
Chapter 9. Phase Diagram II Immiscibility: 不溶性 Spinodal Points:拐点 Simple Eutectic Diagram: 简单共晶相图 Peritectic Phase diagram:包晶相图 Compounds:化合物 Conclusion Remarks Examples
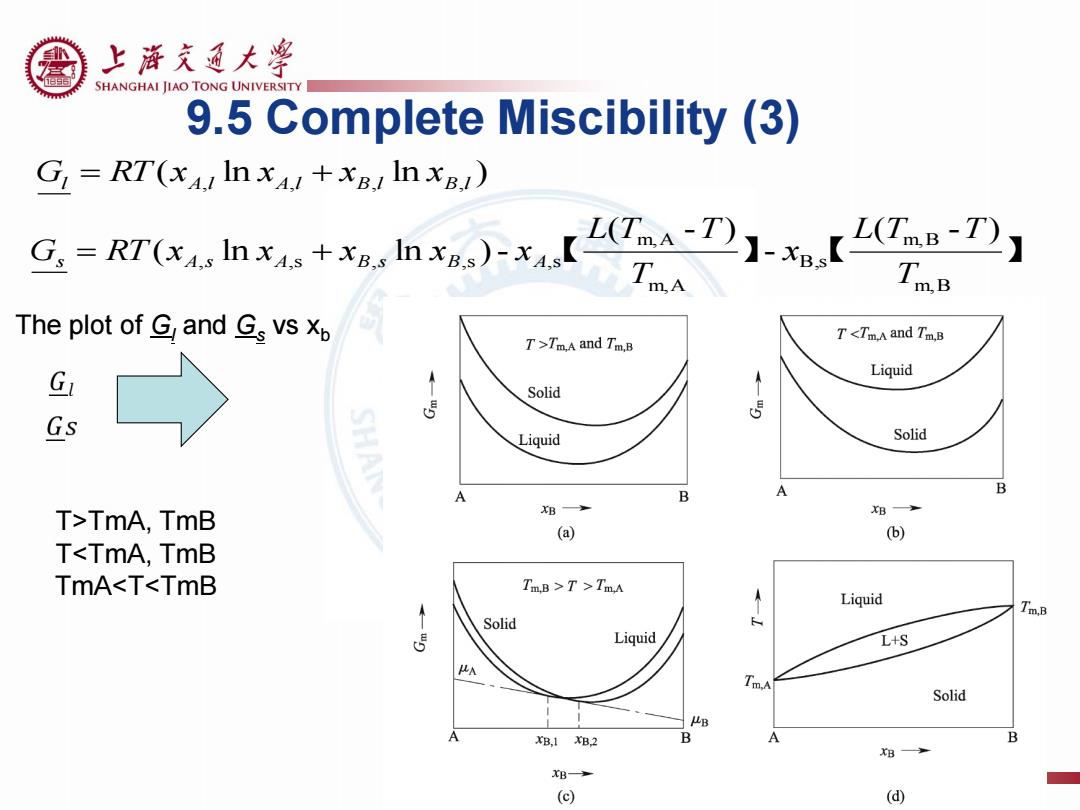
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY 9.5 Complete Miscibility (3) G RT(xAI In xA+xB In xB) uhe+.lh)-x【O】-【 wB The plot of G and Gs vs p T Tm.A and Tm.B G Liquid T Solid 5 5 Gs Liquid Solid B A B T>TmA,TmB B B (a) (b) TT>TmA Liquid Tm.B Solid Liquid L+S A Solid B B,1B2 B A B B B (⊙ (d)
【 】 【 】 m,B m,B B ,s m,A m,A , ,s , ,s ,s ( - ) - ( - ) ( ln ln )- T L T T x T L T T G RT x x x x x s A s A B s B A ( ln ln ) l A,l A,l B,l B,l G RT x x x x 9.5 Complete Miscibility (3) The plot of Gl and Gs vs xb T>TmA, TmB T<TmA, TmB TmA<T<TmB
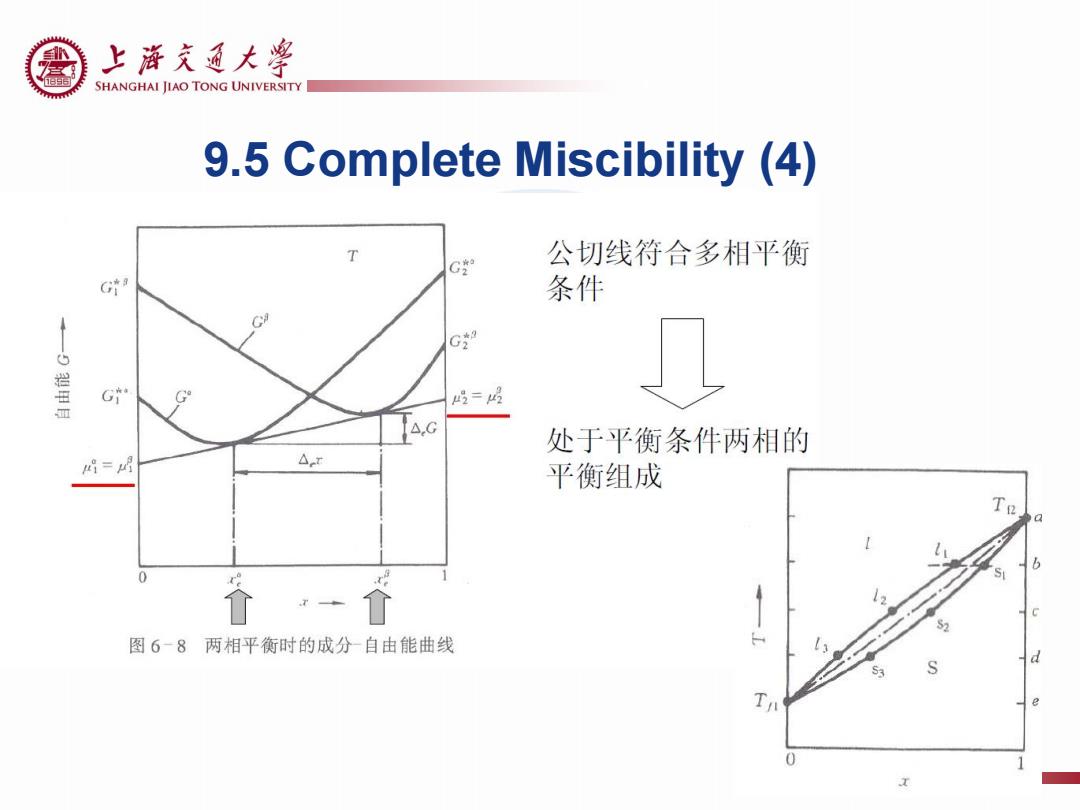
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY 9.5 Complete Miscibility (4) T C° 公切线符合多相平衡 G 条件 C G” G G =喝 处于平衡条件两相的 店= △r 平衡组成 a 图6-8两相平衡时的成分自由能曲线 d Ta
9.5 Complete Miscibility (4)
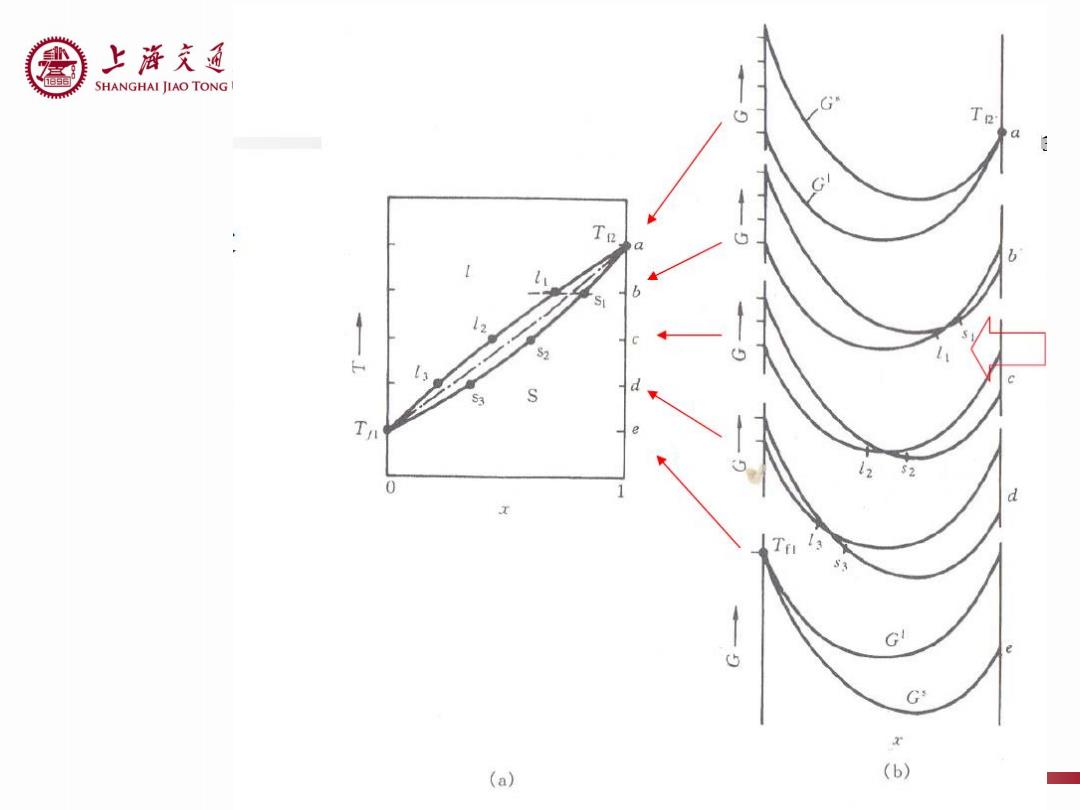
上游充通 SHANGHAI JIAO TONG G Te Te 0 d 1 G 5 G (a) (b)
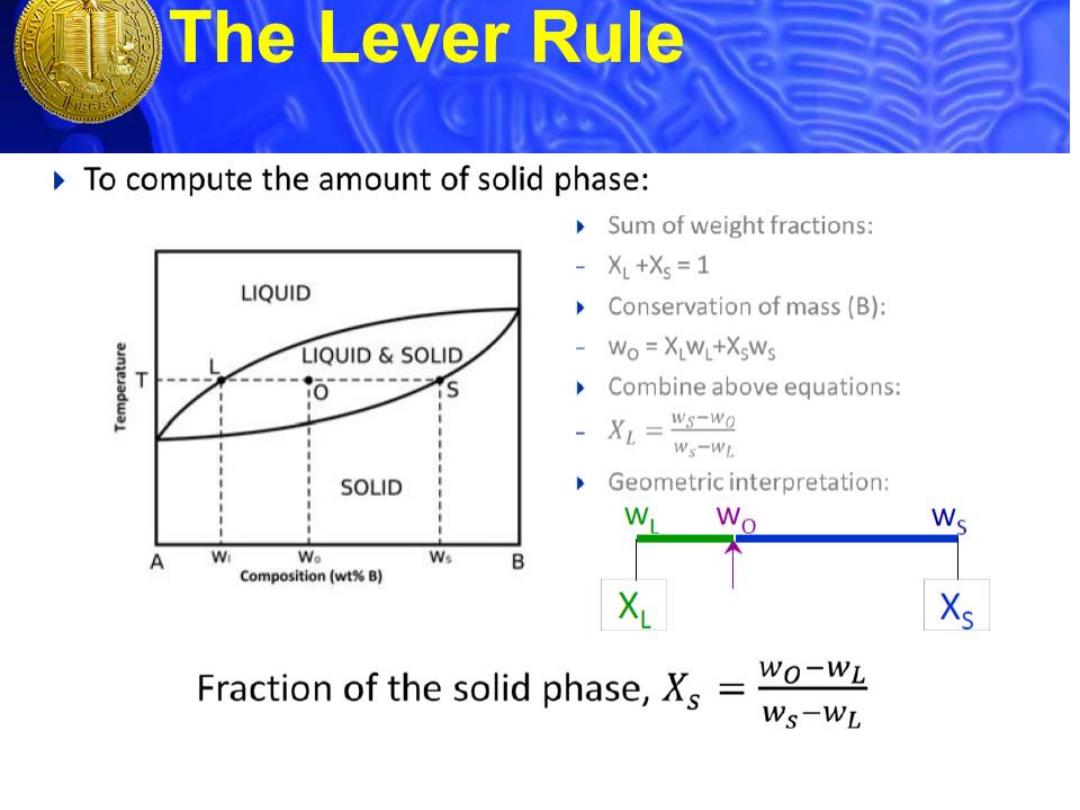
The Lever Rule To compute the amount of solid phase: Sum of weight fractions: XL+Xs =1 LIQUID Conservation of mass(B): LIQUID SOLID Wo XLWL+XsWs T Combine above equations: XL= ws-wo Ws-WL SOLID Geometric interpretation: W Wo Ws W Wo Ws B Composition(wt%B) X Fraction of the solid phase,Xs= Wo-WL Ws-WL
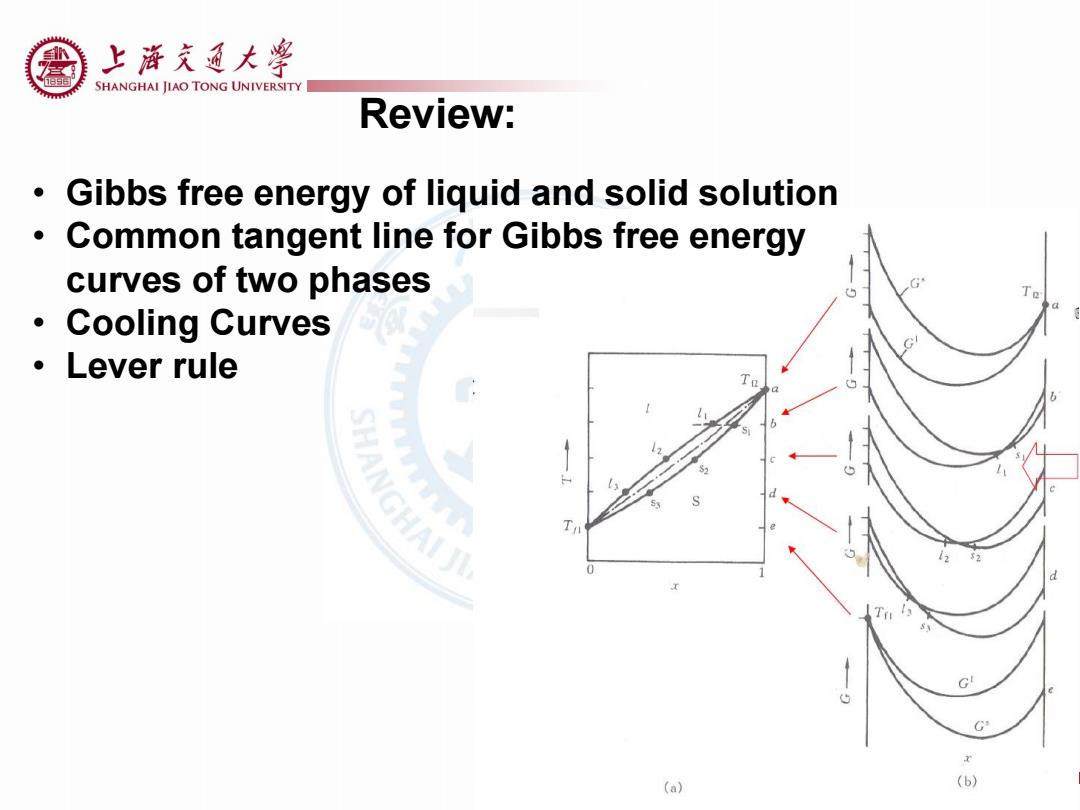
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Review: Gibbs free energy of liquid and solid solution Common tangent line for Gibbs free energy curves of two phases Cooling Curves Lever rule G 5 G (a) (b)
Review: • Gibbs free energy of liquid and solid solution • Common tangent line for Gibbs free energy curves of two phases • Cooling Curves • Lever rule
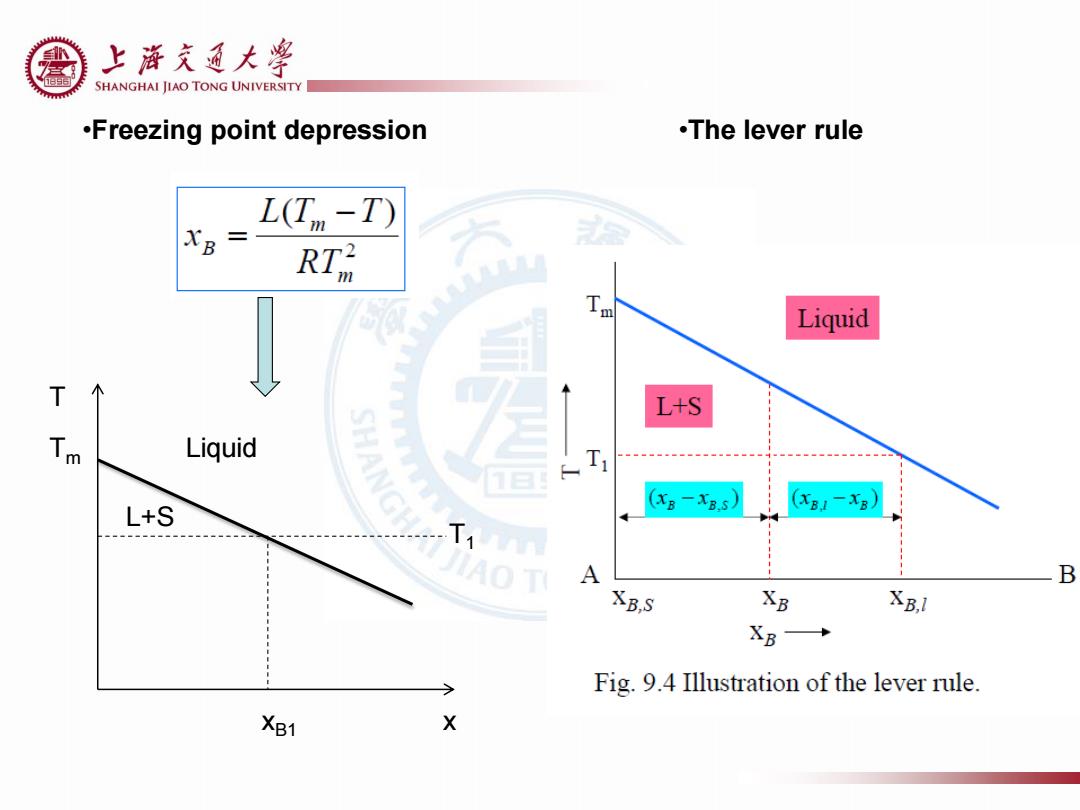
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY .Freezing point depression .The lever rule L(Tn -T) XB RT 人 Liquid T L+S 人 Liquid 12 L+S (XB-xB.s) (B1-x8) AO T A B XB,S XB XB,l XB→ Fig.9.4 Illustration of the lever rule. XB1 X
Tm T Liquid L+S T1 xB1 x •Freezing point depression •The lever rule
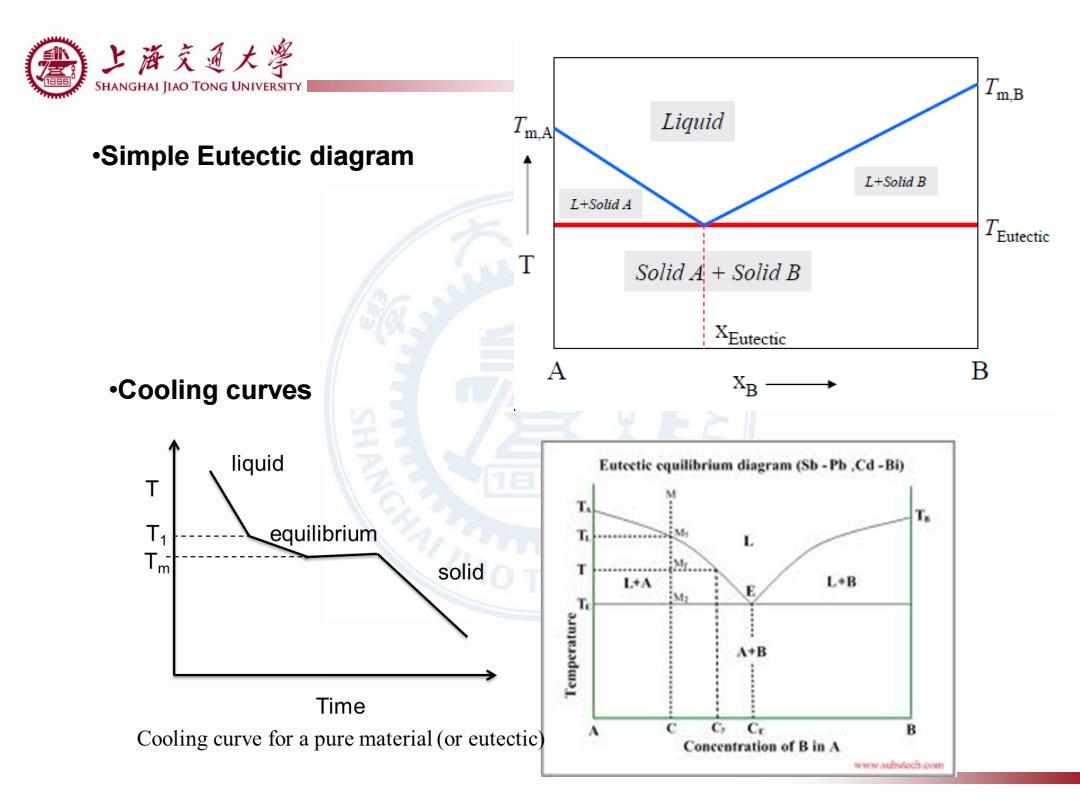
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY TmB Liquid m.A .Simple Eutectic diagram L+Solid B L+SolidA TEutectic T Solid A+Solid B XEutectic A B .Cooling curves B liquid ANG Eutectic cquilibrium diagram (Sb-Pb.Cd-Bi) T equilibrium m solid LA L+B E A+B Time C Cooling curve for a pure material (or eutectic Ce B Concentration of B in A 年wwso边Nm
•Simple Eutectic diagram •Cooling curves
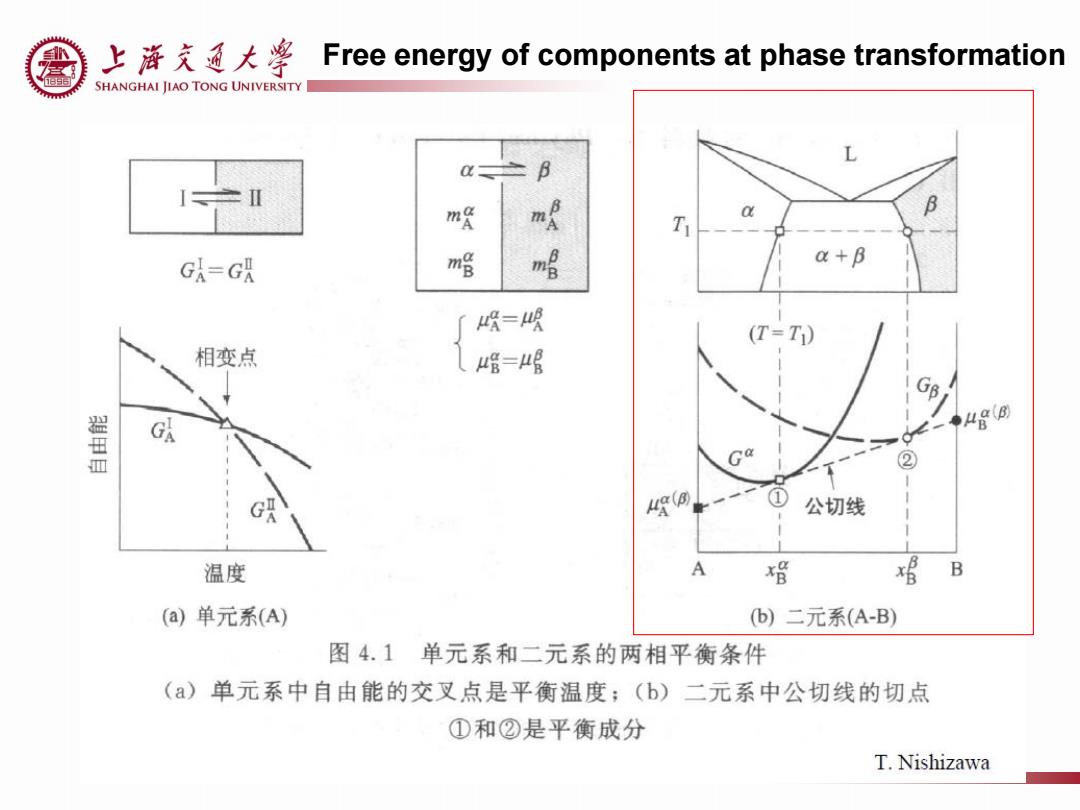
上游充通大¥ Free energy of components at phase transformation SHANGHAI JIAO TONG UNIVERSITY L m a T GA-GA m哈 a+B 喉=喉 (T=T) 相变点 唱=μ唱 ② 公切线 温度 A 磴 B (a)单元系(A) b)二元系(A-B) 图4.1单元系和二元系的两相平衡条件 ()单元系中自由能的交叉点是平衡温度;(b)二元系中公切线的切点 ①和②是平衡成分 T.Nishizawa
Free energy of components at phase transformation
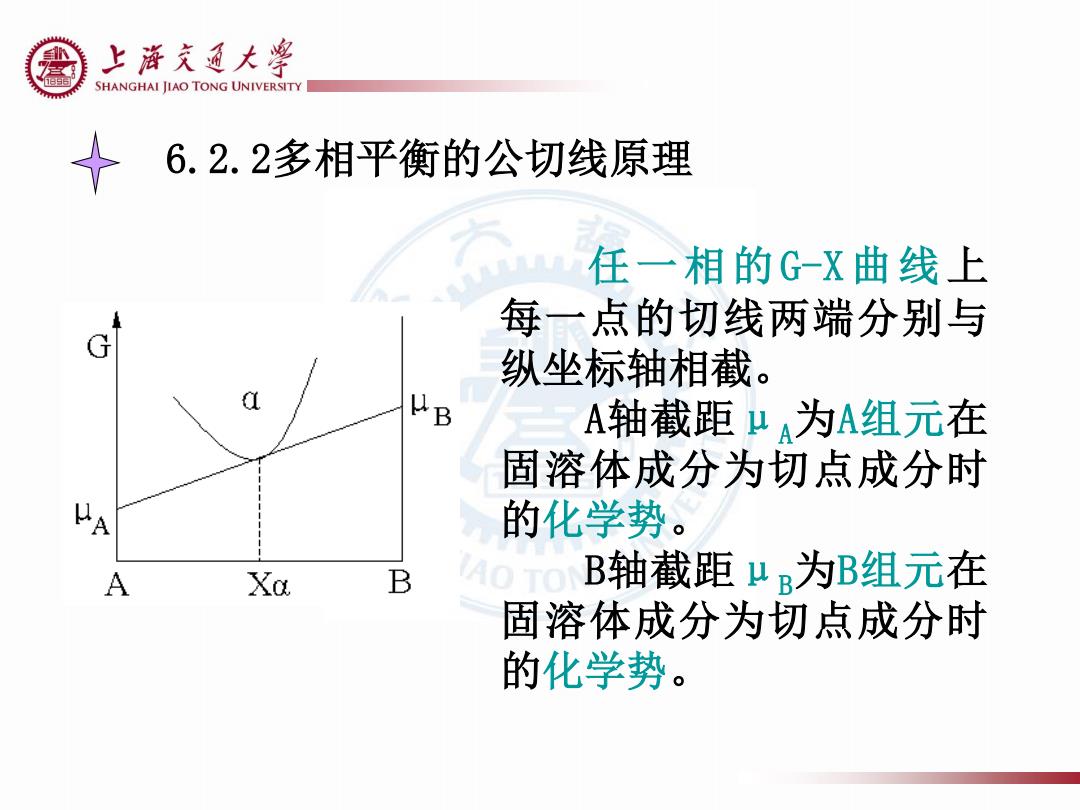
上游克通大粤 e SHANGHAI JIAO TONG UNIVERSITY 6.2.2多相平衡的公切线原理 任一相的G-X曲线上 每一点的切线两端分别与 纵坐标轴相截。 A轴截距μA为A组元在 固溶体成分为切点成分时 的化学势。 A Xa B A0T0 B轴截距μR为B组元在 固溶体成分为切点成分时 的化学势
6.2.2多相平衡的公切线原理 任一相的G-X曲线上 每一点的切线两端分别与 纵坐标轴相截。 A轴截距μA为A组元在 固溶体成分为切点成分时 的化学势。 B轴截距μB为B组元在 固溶体成分为切点成分时 的化学势