
上游克通大粤 SHANGHAI JIAO TONG UNIVERSITY Chapter 8.Phase Rule p/kPa B 水 dp △H dTeg Te9△V (液相) 22090 dp △fsHm Tm①-ms】 △H,a吧 冰 dp▣ dTeg (固相) 蒸发线 1ap0 101.35 汽 0.611 (气相 升华线 0 273.16373.15 647.29 T/K
Chapter 8. Phase Rule
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Nomenclature Phase:相 Component:组元 Degrees of freedom:自由度 Phase rule:相律 Phase diagram:相图 One-component system:单元系
Nomenclature

上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases Phase:a homogeneous,physically distinet,and mechanically separable portion of matter present in a nonhomogeneous physical chemical system. GHAIJIAO TONG UNIVE nm
Phases

上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Phases(2) in thermodynamics,chemically and physically uniform or homogeneous quantity of matter that can be separated mechanically from a nonhomogeneous mixture and that may consist of a single substance or of a mixture of substances.The three fundamental phases of matter are solid,liquid,and gas (vapour),but others are considered to exist,including crystalline,glassy,amorphous,and plasma phases. Thermodynamics Kinetics
Phases (2) Thermodynamics Kinetics

上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases(3) Matter is considered to form one homogeneous phase if its atomic or molecular dispersion is uniform;e.g.,a glass of water containing dissolved salt,sugar,and a dye constitutes only a single liquid phase.If hundreds of grains of sand were added,all the grains together would constitute only a single additional (solid)phase. The different phases of a pure substance bear a fixed relationship to one another in terms of temperature and pressure.Thus,if the pressure on some liquids is raised,they will freeze at a higher temperature.This relationship is extremely important in industrial as well as scientific work. Salt water; Pure water with two pieces of ice on the surface; Gold sands and sands; alloys
Phases (3) Salt water; Pure water with two pieces of ice on the surface; Gold sands and sands; alloys
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Phases Diagram graph showing the limiting conditions for solid. liquid,and gaseous phases of a single substance or of a mixture of substances while undergoing changes in pressure and temperature or in some other combination of variables,such as solubility and temperature. G JIAO TONG UNIV
Phases Diagram
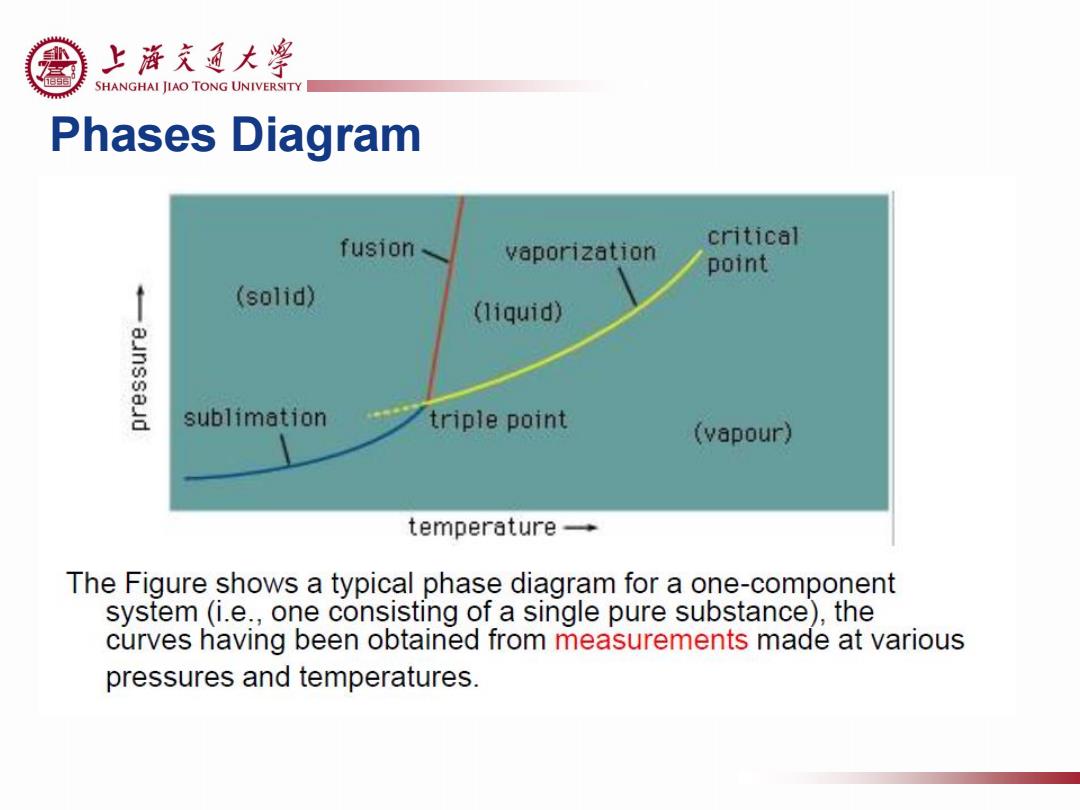
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Phases Diagram fusion、 critical vaporization point (solid) (liquid) sublimation triple point (vapour) temperature- The Figure shows a typical phase diagram for a one-component system(i.e.,one consisting of a single pure substance),the curves having been obtained from measurements made at various pressures and temperatures
Phases Diagram

上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases Rule Thermodynamic stability of coexisting elements,compounds,and solution Equilibrium structure How many phases 所有多相平衡系统都遵循的普遍规律! 描述平衡系统中相数、组分数以及影响系统状态的独立 可变因素(如温度、压力、组成等)的总数(称为自由 度数)之间的关系
Phases Rule

上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY 8.1 Phases 何谓相:物理、化学性质相同的均匀部分 相与相之间有明确界面,越过界面,性质突变 气相,无论多少,1相 液相,纯、溶液(均匀),1相 多种液体混合,溶解度,1,2,3, 固相,纯或原子分子状态相互混合成固溶体,1相 一般体系中,多一种固体便多一个相 Gas:1 phase Liquid:pure liquid or solution=1 phase Mixture of different liquids:more than 1 phases Solid:pure or atomically/molecularly distributed system,1 phase in general,one more solid=1 more phase
8.1 Phases Gas: 1 phase Liquid: pure liquid or solution= 1 phase Mixture of different liquids: more than 1 phases Solid: pure or atomically/molecularly distributed system, 1 phase in general, one more solid= 1 more phase

上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY 8.1 Phases(2) 冰、水 冰、盐水溶液 冰、盐水溶液、盐粒 冰、盐水溶液、平衡气相 Ice,water Ice,salt water Ice,salt water,salt particles Ice ,salt water,equilibrium vapor
8.1 Phases (2) Ice, water Ice, salt water Ice, salt water, salt particles Ice ,salt water, equilibrium vapor