
Contents of Today S.J.T.0. Phase Transformation and Applications Continue /Review previous Entropy 2nd law Heat engine etc. http://smse.sjtu.edu.cn/entropy SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Contents of Today Continue / Review previous Entropy 2nd law Heat engine etc. http://smse.sjtu.edu.cn/entropy
Index of nomenclature S.J.T.0. Phase Transformation and Applications Second Law第二定律 Entropy熵 Reversible process可逆过程 Carnot cycle卡诺循环 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Index of nomenclature Second Law第二定律 Entropy熵 Reversible process可逆过程 Carnot cycle卡诺循环 Index of nomenclature
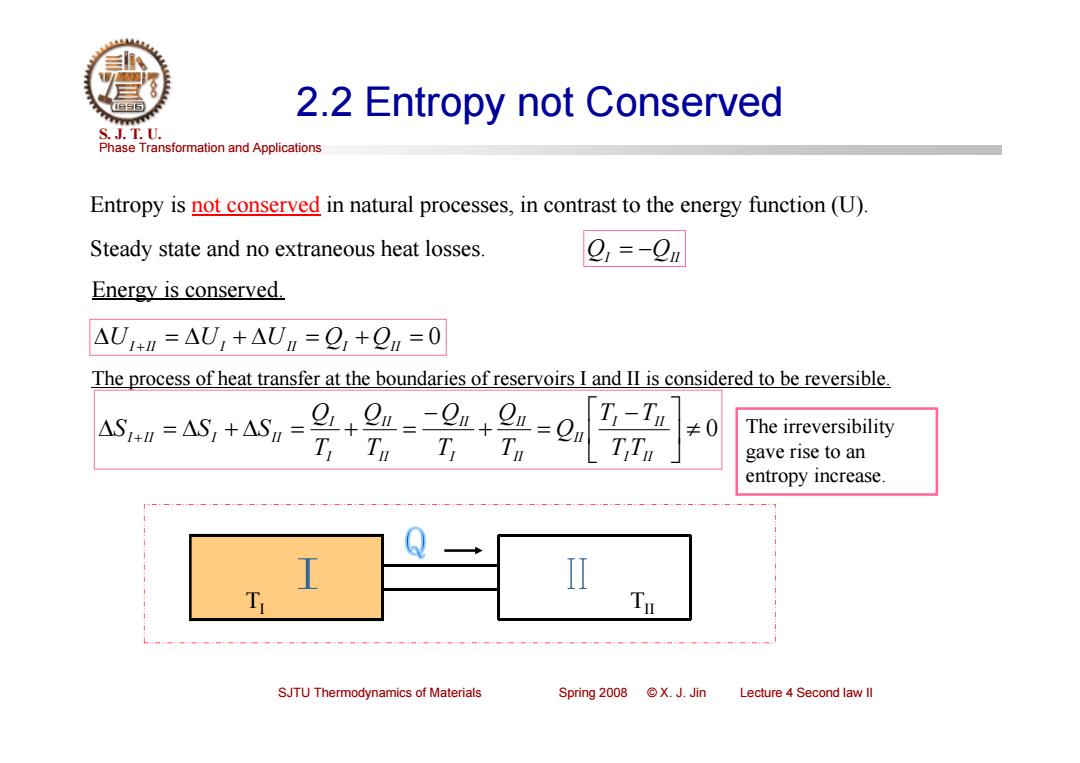
2.2 Entropy not Conserved S.J.T.0. Phase Transformation and Applications Entropy is not conserved in natural processes,in contrast to the energy function(U) Steady state and no extraneous heat losses. 9,=-9u Energy is conserved. AUIn=AU,+AUn=O+On=0 The process of heat transfer at the boundaries of reservoirs I and II is considered to be reversible. AS=AS,+AS=+0m--0n+- T-Tn ≠0 The irreversibility T Tu T gave rise to an entropy increase. T SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II 2.2 Entropy not Conserved Entropy is not conserved in natural processes, in contrast to the energy function (U). TI TII Steady state and no extraneous heat losses. QI QII Energy is conserved. 0 UI II UI UII QI QII The process of heat transfer at the boundaries of reservoirs I and II is considered to be reversible. 0 I II I II II IIII I II IIII II I II I II T T T T Q TQ TQ TQ TQ S S S The irreversibility gave rise to an entropy increase

Reversible Process(2) S.J.T.0. Phase Transformation and Applications 功的计算、可逆过程和最大功 活塞上砝码同时取走三个,外压P,一 次降低到p2,并在p,外压下,气体体积 t口aa 由V,膨胀到V, 活塞上砝码分二次取走,外压由卫,分 段经p,降到p2,气体由',分段经V; ona▣ 膨胀到V2 膨胀过程中,外压始终比系统内压相 差无限小 图1-5不同途径下气体等温膨张示意图 当系统以上述三种途径达到终态后,再各自以其相反的过程回到始态,这就构 成了与原过程方向相反的三种途径。今分别计算其正、逆过程的体积功,分析 比较,引入可逆过程的概念 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Reversible Process (2) 功的计算、可逆过程和最大功 活塞上砝码同时取走三个,外压p1一 次降低到p2,并在p2外压下,气体体积 由V1膨胀到V2 活塞上砝码分二次取走,外压由p1分 段经 p‘; 降到p2,气体由V1分段经 V ‘; 膨胀到V2 膨胀过程中,外压始终比系统内压相 差无限小 当系统以上述三种途径达到终态后,再各自以其相反的过程回到始态,这就构 成了与原过程方向相反的三种途径。今分别计算其正、逆过程的体积功,分析 比较,引入可逆过程的概念

Reversible Process (7) S.J.T.0. Phase Transformation and Annlicatinns 妻1-1不同途径功的比较 途 径 I及I 亚及I Ⅲ及I' W正 -1.87(I) -2.49(亚) -3.46(正) W/KJ W逆 +7.48(1') +4.99(1') +3.46(Ⅲ') W正+W递 +5.61 +2.50 0 由表可知系统由一个状态变化到另一状态,可以通过不同的途径来实现。 当系统状态变化后,再使系统恢复到始态,环境不一定能复原,只有途径 Ⅲ,当系统复原时环境中没有留下功变热的痕迹,此过程为可逆过程。而 另一种情况是系统经过途径、Ⅱ后,系统虽复原,但环境中留下功变热的 痕迹,途径I、Ⅱ为不可逆过程。 当系统由始态I经过某一过程变到终态Ⅱ后,如能使系统再回到原态,同时 也消除了原过程对环境所产生的一切影响,则原来过程称为可逆过程。 反之,某过程进行后,如果用任何方法都不可能使系统和环境完全复原, 则此过程称为不可逆过程。 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Reversible Process (7) 由表可知系统由一个状态变化到另一状态,可以通过不同的途径来实现。 当系统状态变化后,再使系统恢复到始态,环境不一定能复原,只有途径 III,当系统复原时环境中没有留下功变热的痕迹,此过程为可逆过程。而 另一种情况是系统经过途径I、II后,系统虽复原,但环境中留下功变热的 痕迹,途径I、II为不可逆过程。 当系统由始态I经过某一过程变到终态II后,如能使系统再回到原态,同时 也消除了原过程对环境所产生的一切影响,则原来过程称为可逆过程。 反之,某过程进行后,如果用任何方法都不可能使系统和环境完全复原, 则此过程称为不可逆过程
Heat Engine S.J.T.0. Phase Transformation and Annlicatinns A D D B 缸头盖、 凸轮轴气口 福臂 气门封 Pressure 缸头 气门 OA 中缸 曲 箱 C 摩花yes大山4557制寸20066.0W Volume SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Heat Engine
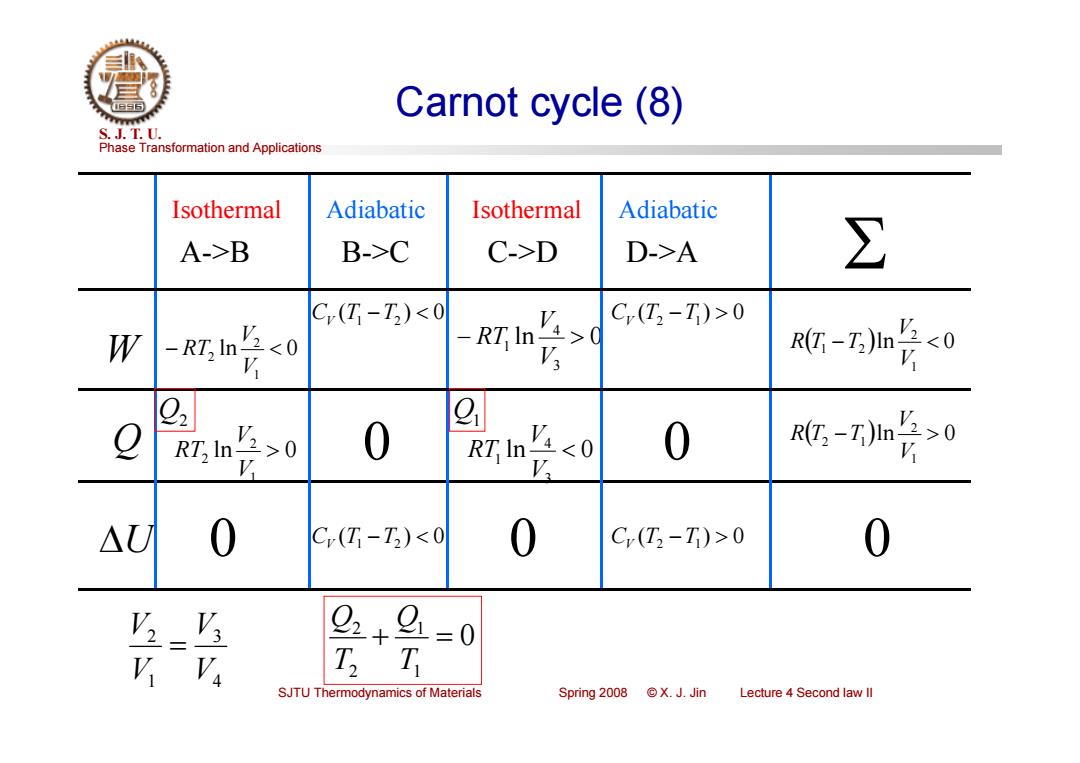
Carnot cycle (8) S.J.T.0. Phase Transformation and Applications Isothermal Adiabatic Isothermal Adiabatic A->B B->C C->D D->A ∑ C(T-T)0 W -RT In V20 RT In V Rt-Z)n点0 0 RT In V,0 0 :-7m长0 △U 0 C(T-T)0 0 2+9 =0 SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Carnot cycle (8) W ln 0 3 4 1 V V Q ln 0 RT 1 2 2 V V RT ln 0 1 2 2 V V RT U 0 A->B B->C C->D D->A 0 Isothermal Isothermal Adiabatic Adiabatic ln 0 3 4 1 V V RT 0 0 CV (T1 T2 ) 0 CV (T2 T1) 0 CV (T1 T2 ) 0 CV (T2 T1) 0 0 ln 0 1 2 1 2 V V R T T ln 0 1 2 2 1 V V R T T Q2 Q1 0 1 1 2 2 T Q T Q 4 3 1 2 V V V V
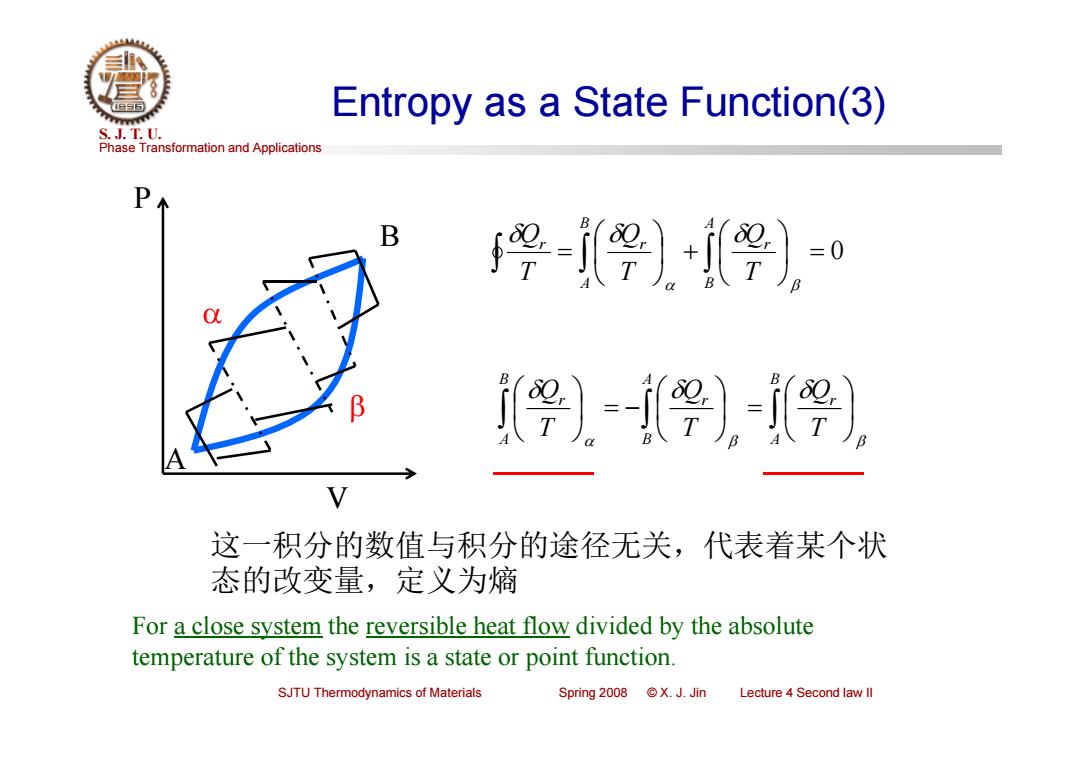
Entropy as a State Function(3) S.J.T.0. Phase Transformation and Applications -))= i〔婴)-婴-i婴) 这一积分的数值与积分的途径无关,代表着某个状 态的改变量,定义为熵 For a close system the reversible heat flow divided by the absolute temperature of the system is a state or point function. SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Entropy as a State Function(3) 0 AB r BA r r TQ TQ TQ BA r AB r BA r TQ TQ TQ P V A B 这一积分的数值与积分的途径无关,代表着某个状 态的改变量,定义为熵 For a close system the reversible heat flow divided by the absolute temperature of the system is a state or point function

The Second Law S.J.T.0. Phase Transformation and Applications The First Law:the conservation of energy and energy transfer in terms of heat and work. Energy is a state function. The Second Law:ENTROPY. Overview Heat engines,devices that convert thermal energy (heat)into mechanical energy (work). The first law places no limits on the amounts that can be converted. The second law is concerned with limits on the conversion of"heat"into s“work”by heat engines. 1824,a French engineer,Sadi Carnot,idealized heat engine. P.W.Atkins,The Second Law,Scientific American Books,1984 SJTU Thermodynamics of Materials Spring2o08©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II The Second Law The First Law: the conservation of energy and energy transfer in terms of heat and work. Energy is a state function. The Second Law: ENTROPY. Overview Heat engines, devices that convert thermal energy (heat) into mechanical energy (work). The first law places no limits on the amounts that can be converted. The second law is concerned with limits on the conversion of “heat” into “work” by heat engines. 1824, a French engineer, Sadi Carnot, idealized heat engine. P. W. Atkins, The Second Law, Scientific American Books, 1984

Measuring the entropy S.J.T.0. Phase Transformation and Applications Display MpIs网 K-1 Entropy Thermometer Monitor of heater SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 4 Second law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 4 Second law II Measuring the entropy