Contents of Today S.J.T.0. Phase Transformation and Applications Review previous Property relation Functions F and G Partial molar quantities Property relation derived from U,H,F,and G etc. SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 6 Property Relation ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II Contents of Today Review previous Property relation Functions F and G Partial molar quantities Property relation derived from U, H, F, and G etc
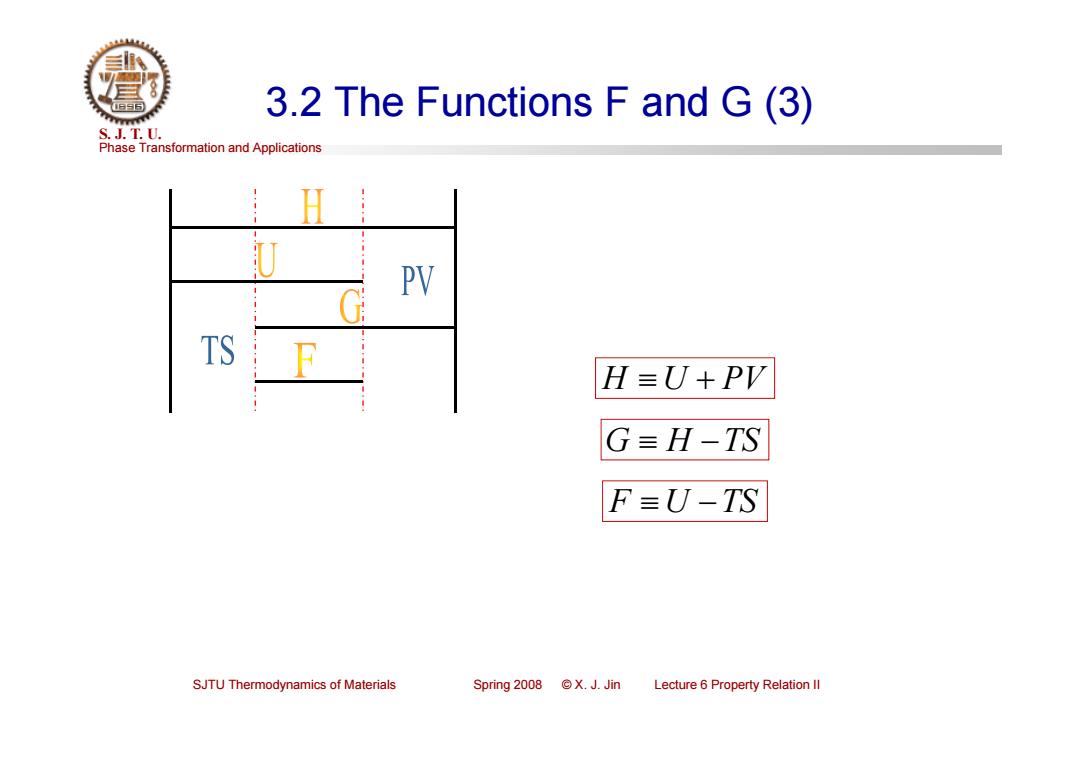
3.2 The Functions F and G(3) S.J.T.0. Phase Transformation and Applications PV TS H=U+PV G=H-TS F=U-TS SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 6 Property Relation ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II 3.2 The Functions F and G (3) F U TS G H TS H U PV
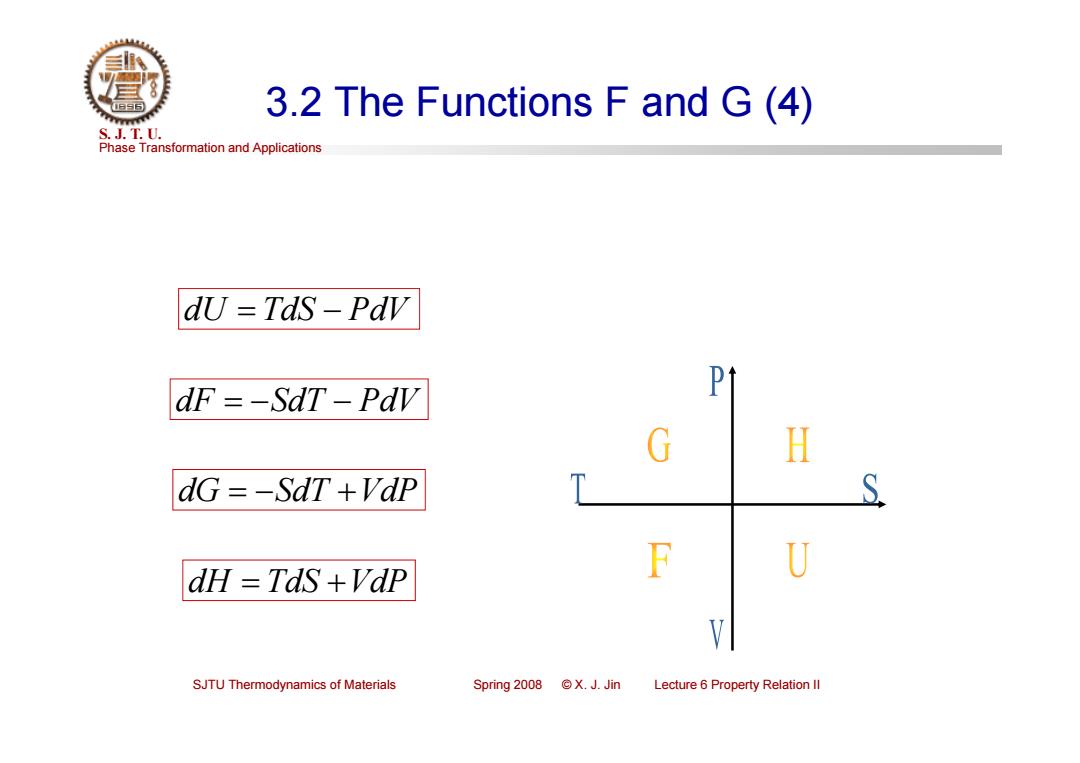
3.2 The Functions F and G(4) S.J.T.0. Phase Transformation and Applications dU =TdS-Pdv dF =-SdT-Pdv Pt G H dG =-SdT +Vdp T S dH TdS +VdP F U V SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 6 Property Relation ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II 3.2 The Functions F and G (4) dU TdS PdV dF SdT PdV dG SdT VdP dH TdS VdP
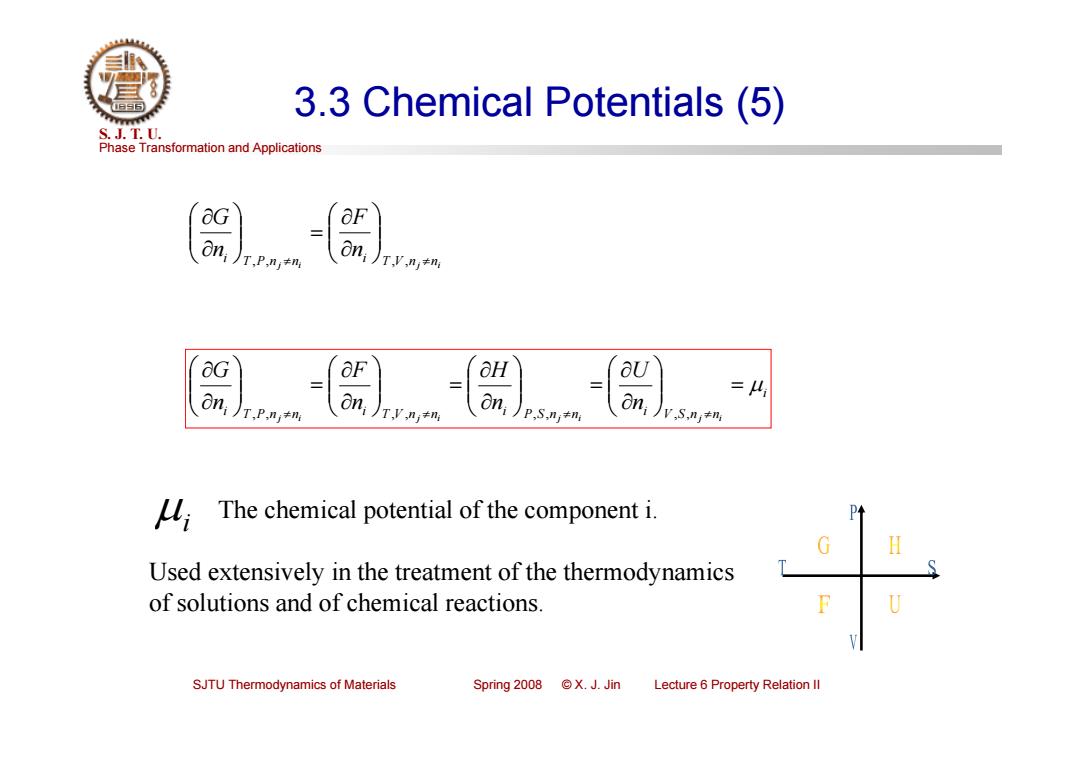
3.3 Chemical Potentials (5) S.J.T.0. Phase Transformation and Applications On, T.P.ni#n T,V,n≠n &G OF aH aU on, On, =4 T.P.ni+n v S,nj+n The chemical potential of the component i. Used extensively in the treatment of the thermodynamics of solutions and of chemical reactions. SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II 3.3 Chemical Potentials (5) j i T V n j ni i T P n n i n F n G , , , , i i T P n j ni i T V n j ni i P S n j ni i V S n j ni n U n H n F n G , , , , , , , , i The chemical potential of the component i. Used extensively in the treatment of the thermodynamics of solutions and of chemical reactions

Review S.J.T.0. Phase Transformation and Applications 当系统由始态I经过某一过程变到终态Ⅱ后,如能使系统再回到原态,同时 也消除了原过程对环境所产生的一切影响,则原来过程称为可逆过程。 H=U+PV dU =TdS-Pdv PV G=H-TS dF =-SdT-PdV TS F=U-TS dG=-SdT +VdP dH TdS +Vdp U The chemical potential of the component i. aG oH aU On, =4 )T,P.nj+m On; T,V,n≠n P,S,n1≠n On: v .S.nj+m SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II Review F U TS G H TS H U PV 当系统由始态I经过某一过程变到终态II后,如能使系统再回到原态,同时 也消除了原过程对环境所产生的一切影响,则原来过程称为可逆过程。 dU TdS PdV dF SdT PdV dG SdT VdP dH TdS VdP i i T P n j ni i T V n j ni i P S n j ni i V S n j ni n U n H n F n G , , , , , , , , i The chemical potential of the component i
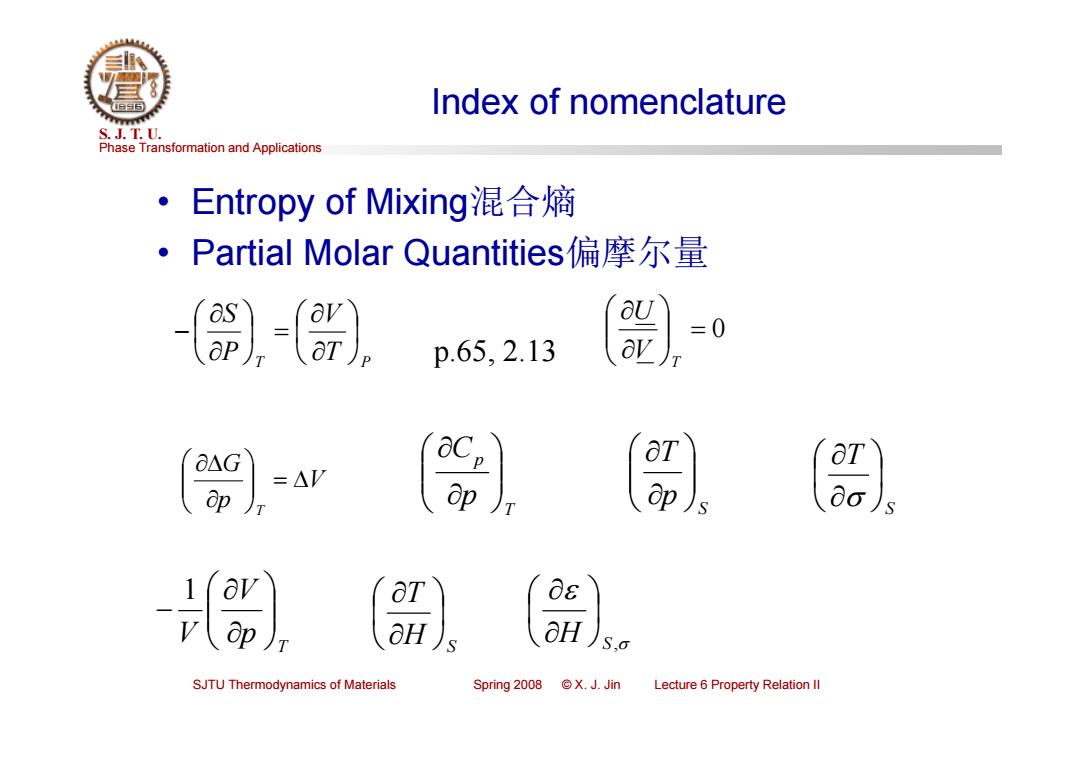
Index of nomenclature S.J.T.0. Phase Transformation and Applications Entropy of Mixing混合熵 ·Partial Molar Quantities偏摩尔量 p.65,2.13 -AV aT ap ap)s ap aH aH S,0 SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II Index of nomenclature • Entropy of Mixing混合熵 • Partial Molar Quantities偏摩尔量 T T P V P S 0 T VU p.65, 2.13 V p G T T p pC S pT S T T p V V 1 H S T H S ,

Partial Molar Quantities S.J.T.0. Phase Transformation and Applications The partial derivative of that quantity with respect to mass(number of moles)at constant temperature and constant pressure,and the mass of all other materials in the system. The partial molar volume of material a in a solution. 7a= av Ona T,P,nb,nc,… Pure a > av The partial molar volume would be equal to the molar volume. Ona ). Constant T and P The chemical potential is the partial molar Gibbs free energy.(Only) na SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II Partial Molar Quantities The partial derivative of that quantity with respect to mass (number of moles) at constant temperature and constant pressure, and the mass of all other materials in the system. a T ,P,nb ,nc , a n V V The partial molar volume of material a in a solution. a T ,P,nb ,nc , a n V Slope V na V Constant T and P Pure a The partial molar volume would be equal to the molar volume. The chemical potential is the partial molar Gibbs free energy. (Only)

Property Relations (1) S.J.T.0. Phase Transformation and Applications OM aN dz Mdx Ndy dU=TdS-Pdv dH TaS +Vap as av dF=-SdT-Pdv as p dG=-SdT+Vap as aP av at H as av SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II Property Relations (1) dz Mdx Ndy dU TdS PdV dH TdS VdP dF SdT PdV dG SdT VdP x y x N y M S S V P V T S S P V P T T T V P V S T T P V P S
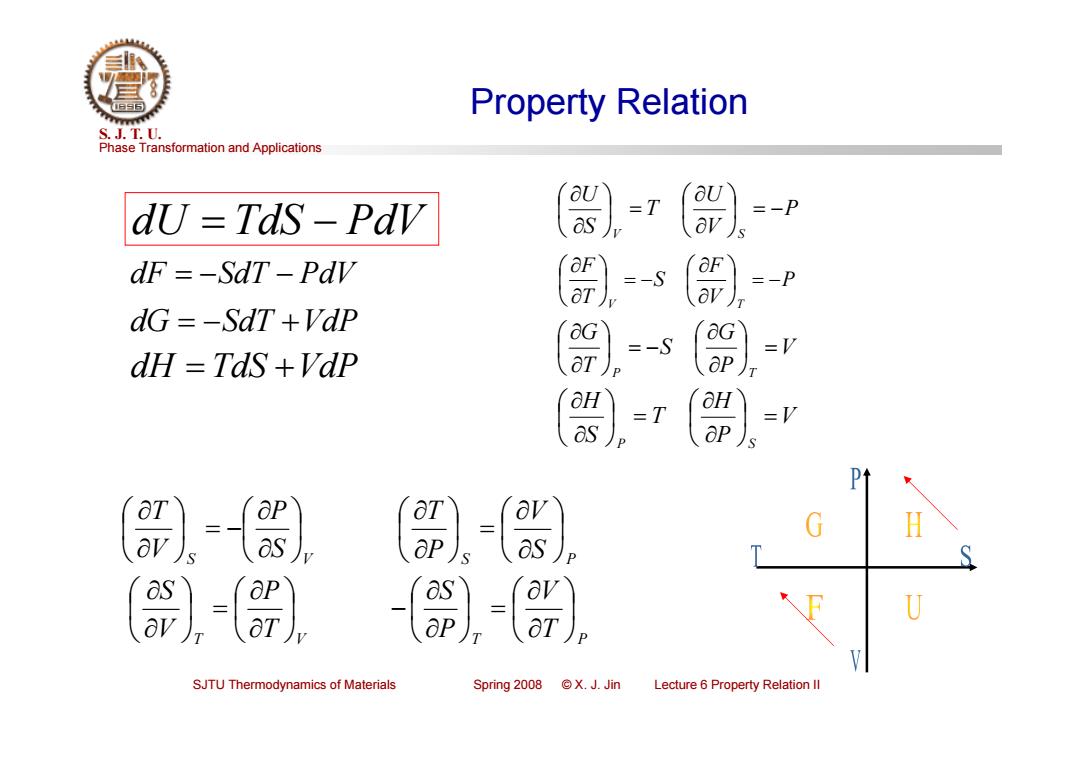
Property Relation S.J.T.0. Phase Transformation and Applications dU=TdS-PdV 〔器) -T =-P dF =-SdT-Pdv 〔) =-S =-P dG =-SdT +Vap =-S =V dH TaS +Vap 〔) -T ap a as aP)s as as U aT) SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II Property Relation S S V P V T S S P V PT T T V P V S T T P V PS dU TdS PdV dF SdT PdV dG SdT VdP dH TdS VdP P V U T S U V S P V F S T F V T V P G S T G P T V P H T S H P S

恒温下熵变的计算(1) S.J.T.0. Phase Transformation and Applications p.65,2.13 s:-s.-fds--(r)dp EX:ideal gas PV=RT R-P ds=- 恒温下,当压力改变时,将引起熵变 For a change in pressure from 1 atm to 10 atm at constant temperature -019.4J/(mol-K) U SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 6 Property Relation II 恒温下熵变的计算(1) T T P V P S 21 21 2 1 PP P dP TV S S d S EX: ideal gas dP P R d S P R P S P R T V PV RT P T For a change in pressure from 1 atm to 10 atm at constant temperature R P R J mol K PdP S RPP ln ln10 19.14 / 101 21 恒温下,当压力改变时,将引起熵变 p.65, 2.13