
上游充通大率 SHANGHAI JIAO TONG UNIVERSITY Principles of Materials Processing Part Ill 全属材料热处理原理 Heat Treatment Principles of Metals Lecturer:Ke Chen 2017-06-02
Principles of Materials Processing Part III - 金属材料热处理原理 Heat Treatment Principles of Metals Lecturer: Ke Chen 2017-06-02

Outline for Chapter 11 上游充道大粤 SHANGHAI JIAO TONG UNIVERSITY Chapter 11:Precipitation transformations 11.1 Precipitation and Aging 11.1.1 Introduction 11.1.2 Continuous Precipitation 11.1.3 Discontinuous Precipitation 11.2 Tempering of Ferrous Martensite AIJIAO TONG UNI K.Chen 2
K. Chen Chapter 11: Precipitation transformations 11.1 Precipitation and Aging 11.1.1 Introduction 11.1.2 Continuous Precipitation 11.1.3 Discontinuous Precipitation 11.2 Tempering of Ferrous Martensite Outline for Chapter 11 2
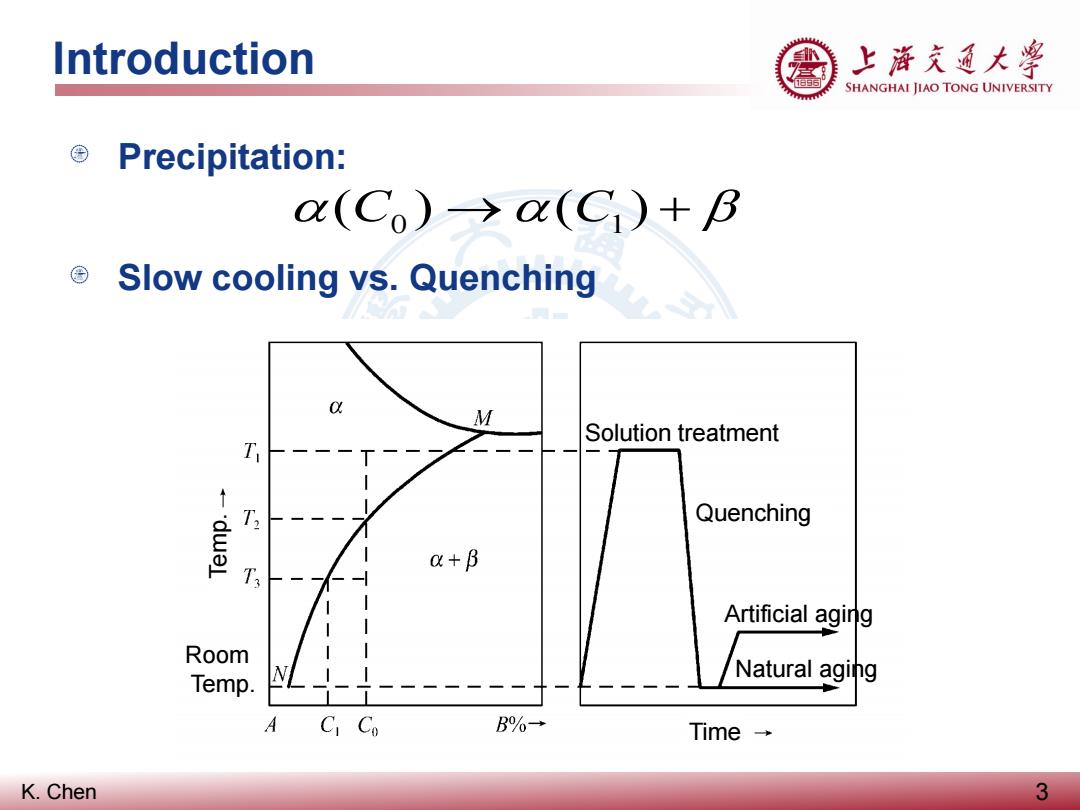
Introduction 上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY ©Precipitation: (Co)→o(C1)+B © Slow cooling vs.Quenching M Solution treatment T Quenching a+B T Artificial aging Room Temp. L Natural aging A CI Co B%→ Time→ K.Chen 3
K. Chen Precipitation: Slow cooling vs. Quenching Introduction 3 α(C0 ) →α(C1) + β Solution treatment Quenching Artificial aging Natural aging Time Room Temp. Temp

Introduction 上游充道大粤 SHANGHAI JIAO TONG UNIVERSITY Ageing(时效): ■ When a precipitation hardening alloy is quenched after solution treatment,its alloying elements will be trapped in solution,resulting in a soft metal. ■ Aging a“solutionized”(固溶处理)metal will allow the alloying elements to diffuse through the microstructure and form intermetallic particles. Natural aging(自然时效):aging at room temperature Artificial aging(人工时效):aging at elevated temperature K.Chen 4
K. Chen Ageing (时效): When a precipitation hardening alloy is quenched after solution treatment, its alloying elements will be trapped in solution, resulting in a soft metal. Aging a “solutionized” (固溶处理) metal will allow the alloying elements to diffuse through the microstructure and form intermetallic particles. Natural aging (自然时效): aging at room temperature Artificial aging (人工时效): aging at elevated temperature Introduction 4
Introduction 上游充道大粤 SHANGHAI JIAO TONG UNIVERSITY ©→Precipitation hardening(沉淀强/硬化)or ageing hardening(时效强/硬化) Continuous precipitation vs.Discontinuous precipitation ■ Continuous (precipitation (or general precipitation): it occurs generally throughout the matrix on dislocations or grain boundaries,etc.and the composition of matrix surrounding the precipitates decreases continuously with time Discontinuous(不连续)precipitation(or cellular precipitation,胞状脱溶):the composition of the matriⅸ changes discontinuously as the cell front passes K.Chen 5
K. Chen Precipitation hardening (沉淀强/硬化) or ageing hardening (时效强/硬化) Continuous precipitation vs. Discontinuous precipitation Continuous (连续) precipitation (or general precipitation): it occurs generally throughout the matrix on dislocations or grain boundaries, etc. and the composition of matrix surrounding the precipitates decreases continuously with time Discontinuous (不连续) precipitation (or cellular precipitation, 胞状脱溶): the composition of the matrix changes discontinuously as the cell front passes Introduction 5
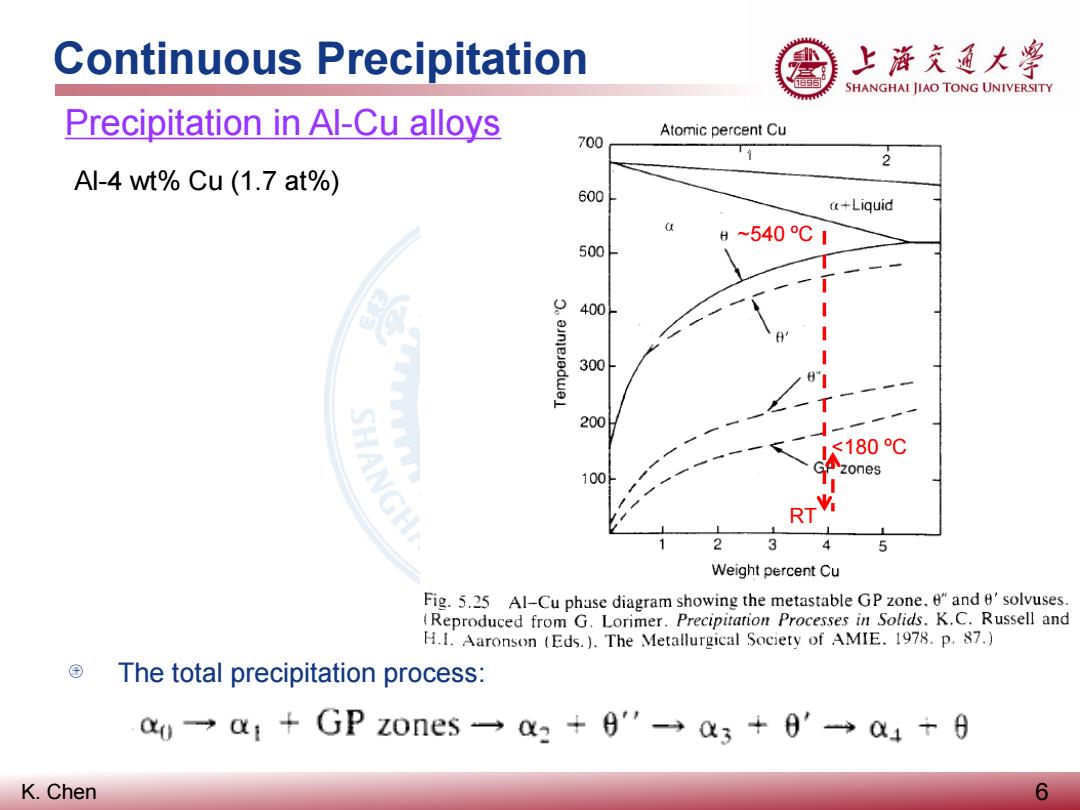
Continuous Precipitation 上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Precipitation in Al-Cu alloys Atomic percent Cu 700 2 Al-4 wt%Cu (1.7 at%) 600 a+Liquid H~540℃1 500 400 300 200 SHANGH <180℃ 100 zones RT 2 3 5 Weight percent Cu Fig.5.25 Al-Cu phase diagram showing the metastable GP zone.e"and e'solvuses. (Reproduced from G.Lorimer.Precipitarion Processes in Solids.K.C.Russell and H.1.Aaronson (Eds.).The Metallurgical Society of AMIE.1978.p.87.) The total precipitation process: a0一a1+GPz0nes一a3+9'-→a3+0'a4+6 K.Chen 6
K. Chen Continuous Precipitation 6 Precipitation in Al-Cu alloys The total precipitation process: Al-4 wt% Cu (1.7 at%) RT <180 ºC ~540 ºC
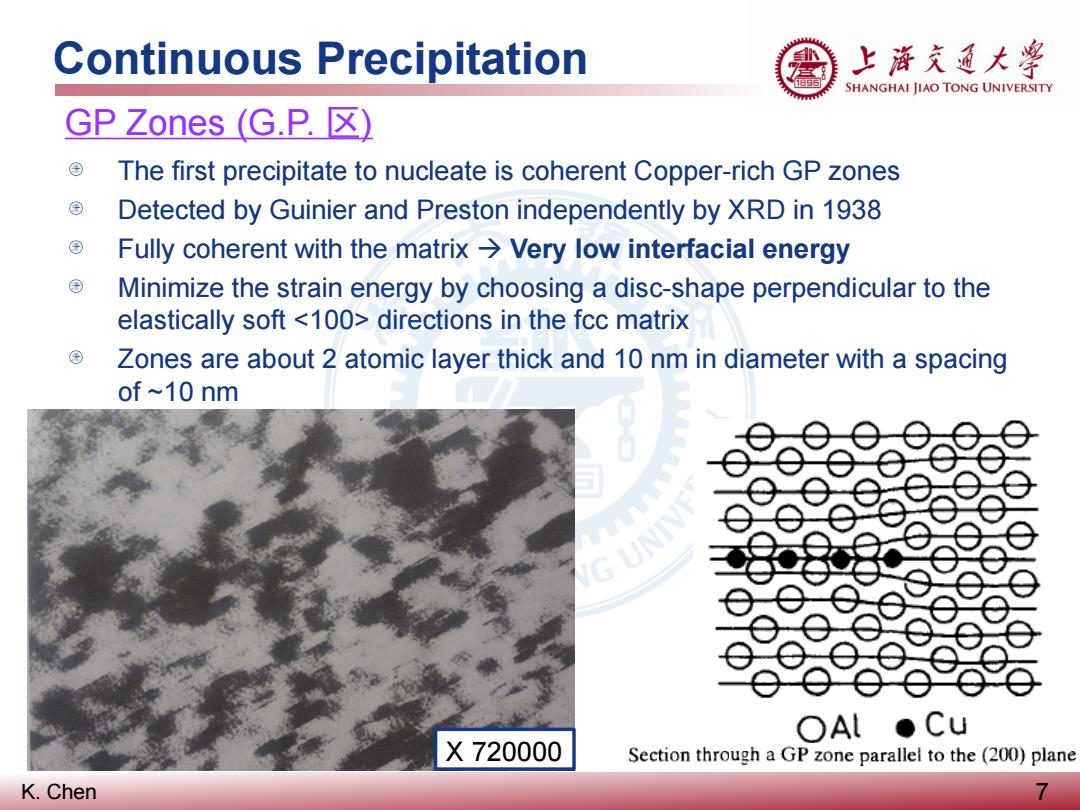
Continuous Precipitation 上游充通大兽 SHANGHAI JIAO TONG UNIVERSITY GP Zones(G.P.区) The first precipitate to nucleate is coherent Copper-rich GP zones Detected by Guinier and Preston independently by XRD in 1938 Fully coherent with the matrix>Very low interfacial energy Minimize the strain energy by choosing a disc-shape perpendicular to the elastically soft directions in the fcc matrix ⊕ Zones are about 2 atomic layer thick and 10 nm in diameter with a spacing of ~10 nm 230列 9e9 G UNIV OAl●Cu X720000 Section through a GP zone parallel to the (200)plane K.Chen 7
K. Chen Continuous Precipitation 7 GP Zones (G.P. 区) The first precipitate to nucleate is coherent Copper-rich GP zones Detected by Guinier and Preston independently by XRD in 1938 Fully coherent with the matrix Very low interfacial energy Minimize the strain energy by choosing a disc-shape perpendicular to the elastically soft directions in the fcc matrix Zones are about 2 atomic layer thick and 10 nm in diameter with a spacing of ~10 nm X 720000

Continuous Precipitation 上游充通大兽 SHANGHAI JIAO TONG UNIVERSITY GP Zones(G.P.区) GP zones resolved by the coherency misfit strain Homogeneous nucleation First precipitate formed during low-T ageing Not only in Al-Cu,but also Al-Zn and Al-Ag alloys X720000 K.Chen 8
K. Chen Continuous Precipitation 8 GP Zones (G.P. 区) GP zones resolved by the coherency misfit strain Homogeneous nucleation First precipitate formed during low-T ageing Not only in Al-Cu, but also Al-Zn and Al-Ag alloys X 720000
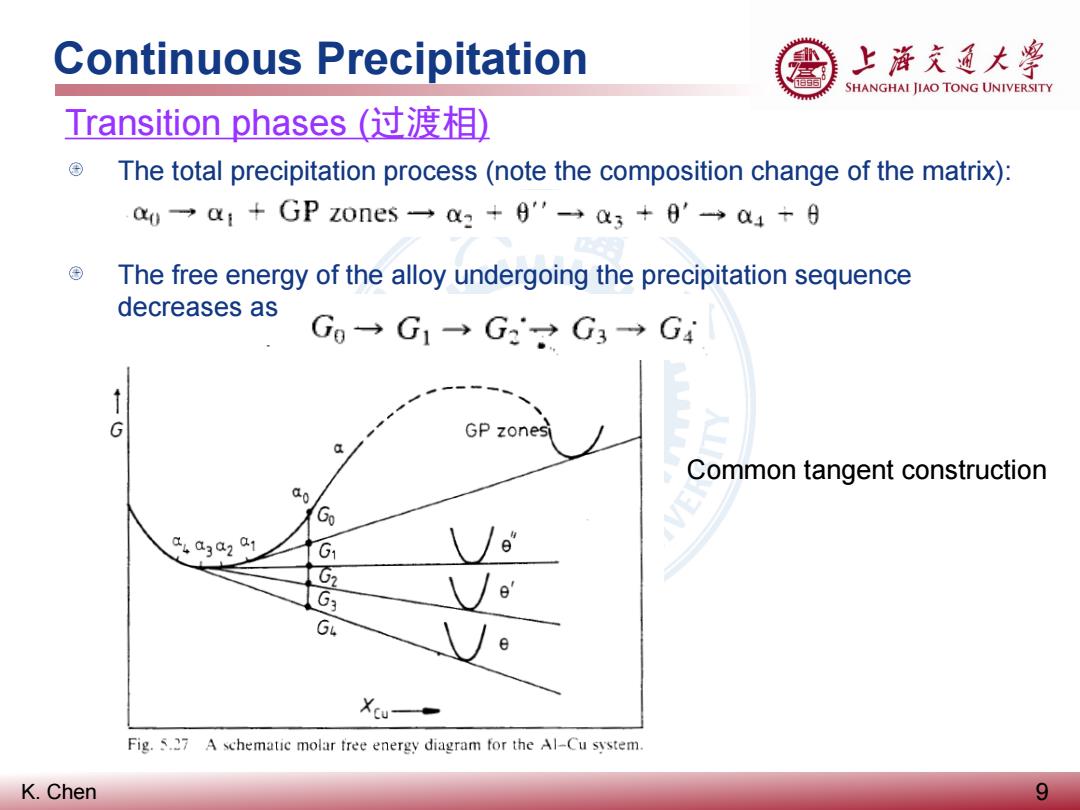
Continuous Precipitation 上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Transition phases(过渡相) The total precipitation process(note the composition change of the matrix): a0一a1+GPZ0ne5一a2+6'-→3+0'a4+9 ⊕ The free energy of the alloy undergoing the precipitation sequence decreases as Gg→G1→G2G3→G4 G GP zonesi Common tangent construction 00 G 4a3a201 G1 \/ G2 G3 o' GL Fig.5.27 A schematic molar free energy diagram for the Al-Cu system. K.Chen 9
K. Chen Continuous Precipitation 9 Transition phases (过渡相) The total precipitation process (note the composition change of the matrix): The free energy of the alloy undergoing the precipitation sequence decreases as Common tangent construction

Continuous Precipitation 上游充通大兽 SHANGHAI JIAO TONG UNIVERSITY Transition phases(过渡相) Transition phases form because they have a lower activation energy barrier for nucleation than the equilibrium phase. ■ Crystal structures of the transition phases are intermediate between those of the matrix and the equilibrium phase>a higher degree of coherence a low interfacial energy contribution to AG*. The equilibrium phase:complex crystal structure>incompatible with the matrix Longer time Total needed △G Free energy size -h G oa+GP GP a,+GPa G2 G3 g+8 Time (a) (b) Fig.5.28 (a)The activation energy barrier to the formation of each transition phase is very small in comparison to the barrier against the direct precipitation of the equilibrium phase.(b)Schematic diagram showing the total free energy of the alloy v.time. K.Chen 10
K. Chen Continuous Precipitation 10 Transition phases (过渡相) Transition phases form because they have a lower activation energy barrier for nucleation than the equilibrium phase. Crystal structures of the transition phases are intermediate between those of the matrix and the equilibrium phase a higher degree of coherence a low interfacial energy contribution to ΔG*. The equilibrium phase: complex crystal structure incompatible with the matrix Longer time needed