
Outline for Chapter 9 图 上帝支是人学 Chapter 9:Eutectoid and Reverse Eutectoid 9.1 Reverse Eutectoid Transformations 9.1.1 Intro to austenite in Fe-C system 9.1.2 Formation mechanism of austenite 9.1.3 Kinetics of austenitization 9.1.4 Austenite grain growth and its control 9.2 Eutectoid Transformations K.Chen 3
3

Glossary 图 上海哀通大学 Homogeneous/Homogeneity Spontaneous growth Inhomogeneous/Inhomogeneity Isothermal Heterogeneous/Heterogeneity √Lamella/Lamellae Eutectoid/Reverse eutectoid Lamellar/Interlamellar Ferrite/Cementite Migration Pearlite/Spheroidite Residual/Remaining Austenite/Austenitization Incubation time Normalizing √Proeutectoid phase Full annealing Hypereutectoid Interstice A0 √Hypoeutectoid Octahedral/Octahedron Tetrahedral/Tetrahedron Orthorhombic Concentration gradient K.Chen 4
4

Intro 上帝支通人学 JLAO TONG UNIN Typical processes for eutectoid transformation: 。Normalizing(正火) Heating at least 55C above the upper critical temperature-i.e., above A for compositions less than the eutectoid (0.76 wt%C).and above Accm for compositions greater than the eutectoid.After sufficient time has been allowed for the alloy to completely transform to austenite-the treatment is terminated by cooling in air Full Annealing(退: Heating to a temperature of about 50C above Ac3(to form austenite) for compositions less than the eutectoid,or,for compositions in excess of the eutectoid.50C above the A,line (to form austenite and Fe3C phases);then furnace cooled(for several hours) For increased machinability and formability In both processes,austenitization occurs before eutectoid reactions a +Fe3C-Y K.Chen 5
5
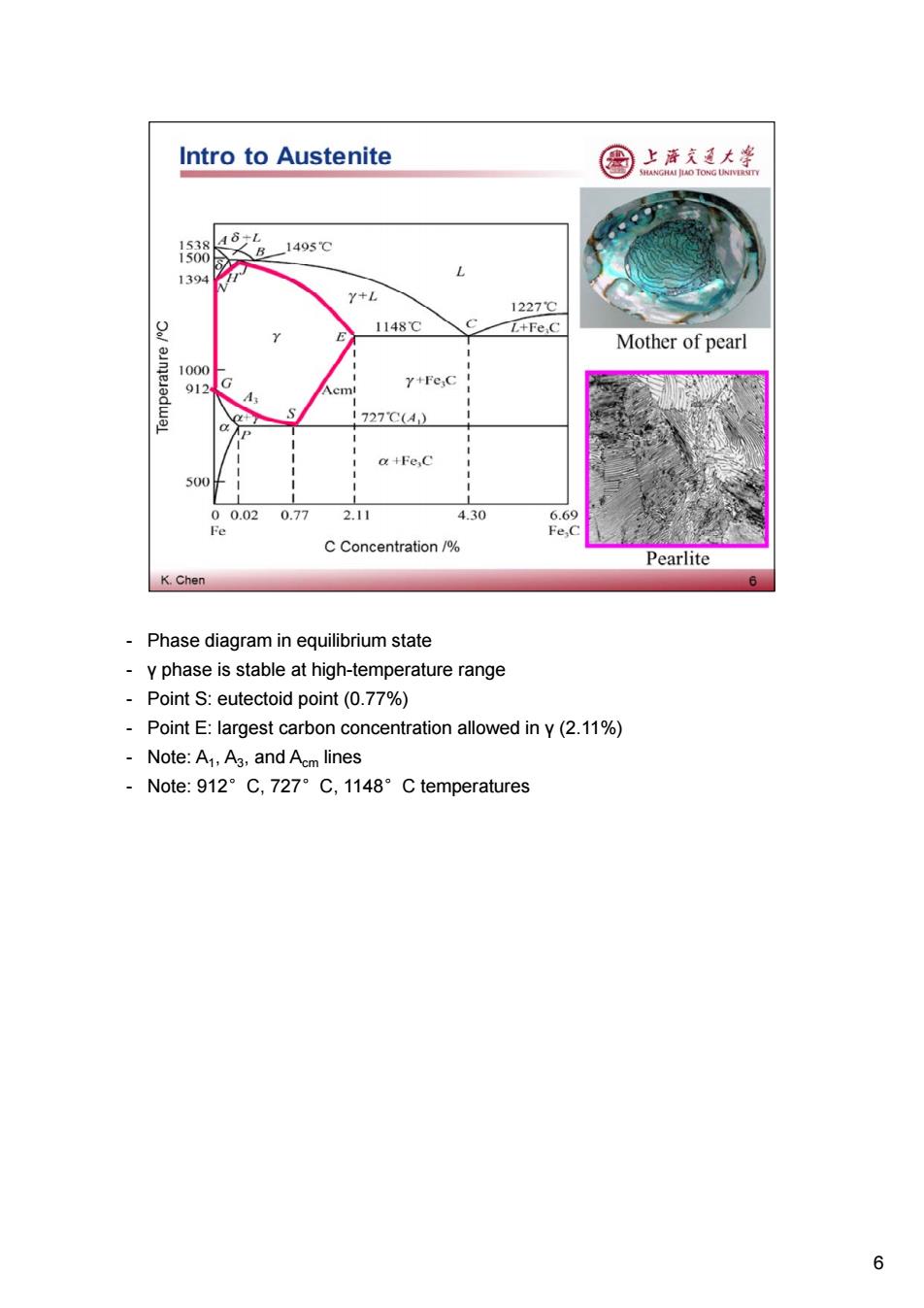
Intro to Austenite 图 上海充通大学 HAI JIAO TONG UNIVI 1538 AδL 1495℃ 1500 1394 Y+L 1227℃ 皇 1148℃ L+Fe C Mother of pearl 1000 912 Y+Fe C cm a+ 1727C(A) a +Fe,C 500 00.020.77 2.11 4.30 6.69 Fe Fe,C C Concentration/% Pearlite K.Chen 6 Phase diagram in equilibrium state y phase is stable at high-temperature range Point S:eutectoid point(0.77%) Point E:largest carbon concentration allowed in y(2.11%) Note:A1,A3,and Acm lines -Note:912°C,727°C,1148°C temperatures 6
- Phase diagram in equilibrium state - γ phase is stable at high-temperature range - Point S: eutectoid point (0.77%) - Point E: largest carbon concentration allowed in γ (2.11%) - Note: A1, A3, and Acm lines - Note: 912°C, 727°C, 1148°C temperatures 6

Intro to Austenite 图 上游充通大学 AI JLAO TONG UNI Actual transformation temperatures: Heating:Aci,Ac3.Accm a+yG Cooling:Ar,Ar3,Arcm Y+FeC Ac stands for arret chauffant(stop heating) A,stands for arret refroidissement(stop +Fe.C cooling) Q weX 100 K.Chen In practice,there is a temperature delay. Degree of delay depends on the heating/cooling rate.Higher rate results in higher degree of delay. 7
- In practice, there is a temperature delay. - Degree of delay depends on the heating/cooling rate. Higher rate results in higher degree of delay. 7
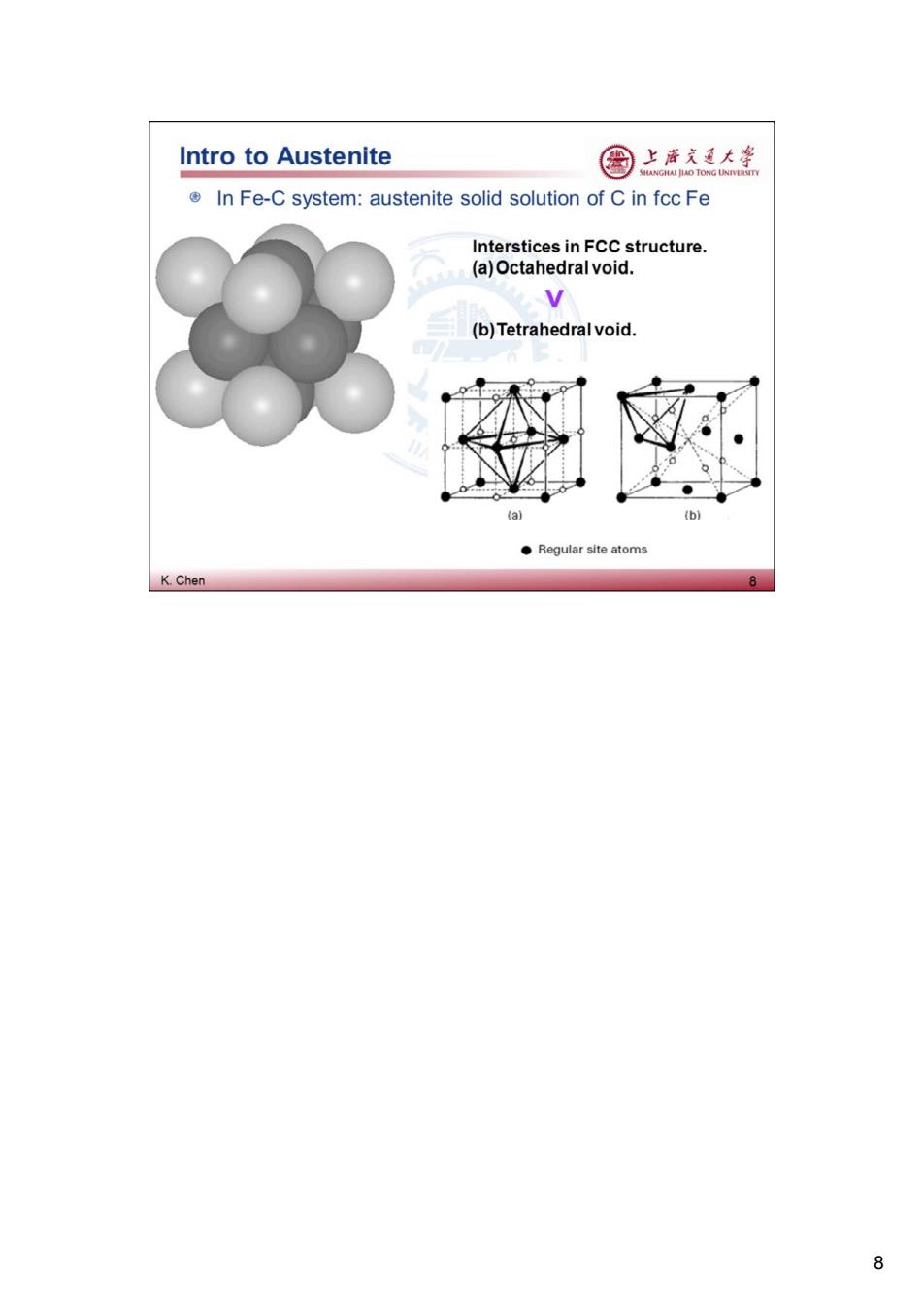
Intro to Austenite 图 上唐东大学 In Fe-C system:austenite solid solution of C in fcc Fe Interstices in FCC structure. (a)Octahedral void. v (b)Tetrahedral void. (a) (b) ●Regular site atoms K.Chen 8 8
8
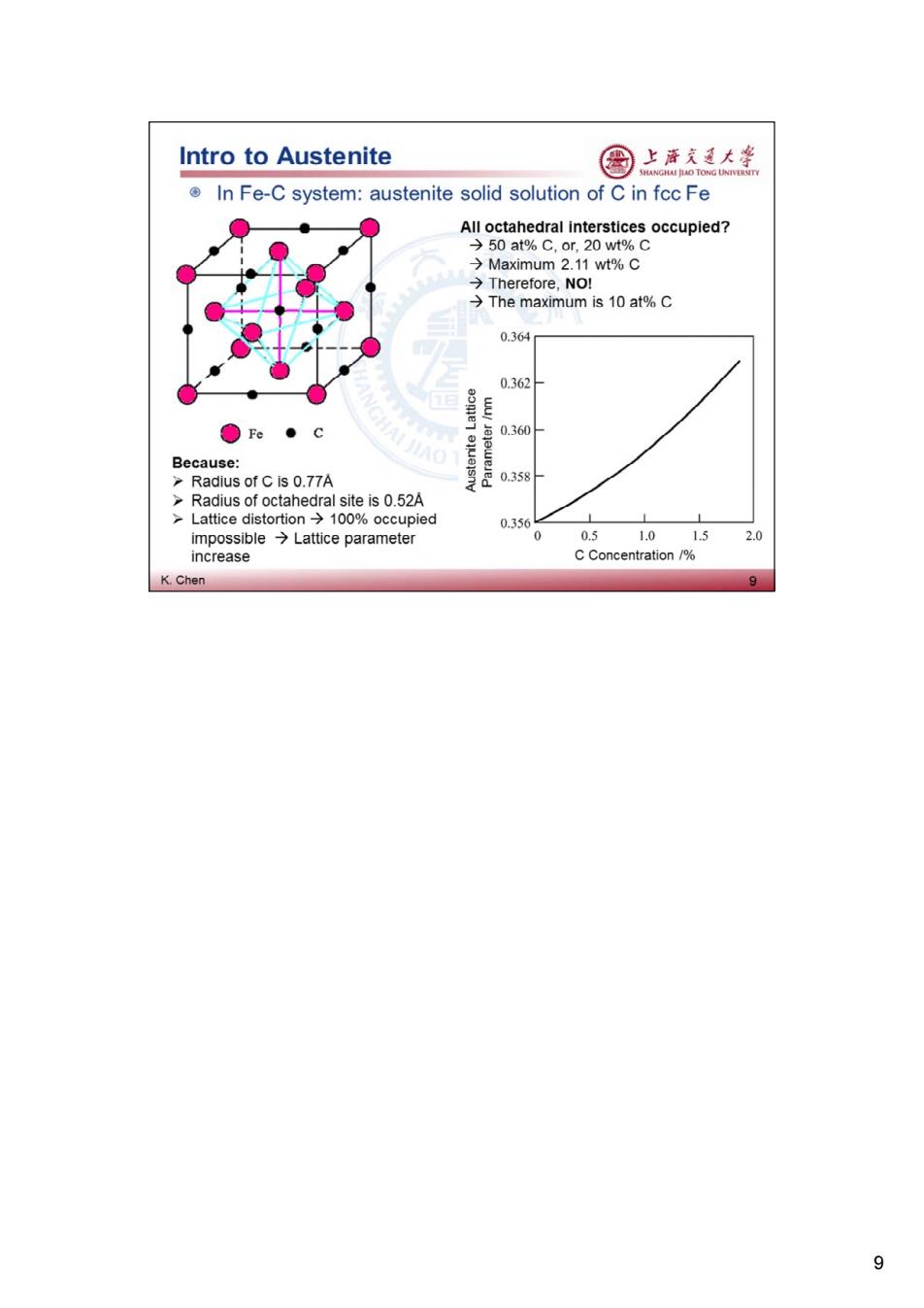
Intro to Austenite 国 上清东通大学 In Fe-C system:austenite solid solution of C in fcc Fe All octahedral interstices occupied? →50at%C,or.20wt%C →Maximum2.11wt%C →Therefore,NoI →The maximum is10at%C 0.364 0.362 Fe●C Because: GHAI JIAO 0.360- >Radius of C is 0.77A 0.358 >Radius of octahedral site is 0.52A >Lattice distortion>100%occupied 0.356 impossible Lattice parameter 0 0.5 1.01.5 2.0 increase C Concentration/% K.Chen 9 9
9

Intro to Austenite 上濟充通大学 HAI JIAO TONG UNIV Properties: Stabilizable to produce Austenitic Steel FCC>low hardness and yield strength Fcc→good formability ©Fcc→most closely packed→Smallest specific volume Small self-diffusion coefficient of Fe>high-temperature application Paramagnetic>non-magnetic steel Large liner expansion coefficient ● Poor thermal conductivity>heating/cooling rate? O TONG UN K.Chen Fe-C phase diagram can be modified by alloying element additions> austenite phase field increases so much that y phase stable at room temperature. In fcc,existence of many slip systems leads to easy dislocation movement. At high heating/cooling rate,large temperature gradient along with large liner expansion coefficient will result in huge thermal stressing inside of workpiece. 10
- Fe-C phase diagram can be modified by alloying element additions austenite phase field increases so much that γ phase stable at room temperature. - In fcc, existence of many slip systems leads to easy dislocation movement. - At high heating/cooling rate, large temperature gradient along with large liner expansion coefficient will result in huge thermal stressing inside of workpiece. 10

Formation Mechanism 上濟充通大学 Transformation temperature:(>Ac) Phases: a Fe3C → Compositions (wt%):0.02% 6.7% 0.77% Crystal structures: BCc Orthorhombic FCC The austenitization involves crystal structure change,dissolution of cementite,and the diffusion of C. AO TONG UNIVERST K.Chen Taking eutectoidal steel as an example to study the formation mechanism of austenite. Orthorhombic:all three bases intersect at 90 angles,so the three lattice vectors remain mutually orthogonal;a,b,and c are distinct. 11
- Taking eutectoidal steel as an example to study the formation mechanism of austenite. - Orthorhombic: all three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal; a, b, and c are distinct. 11
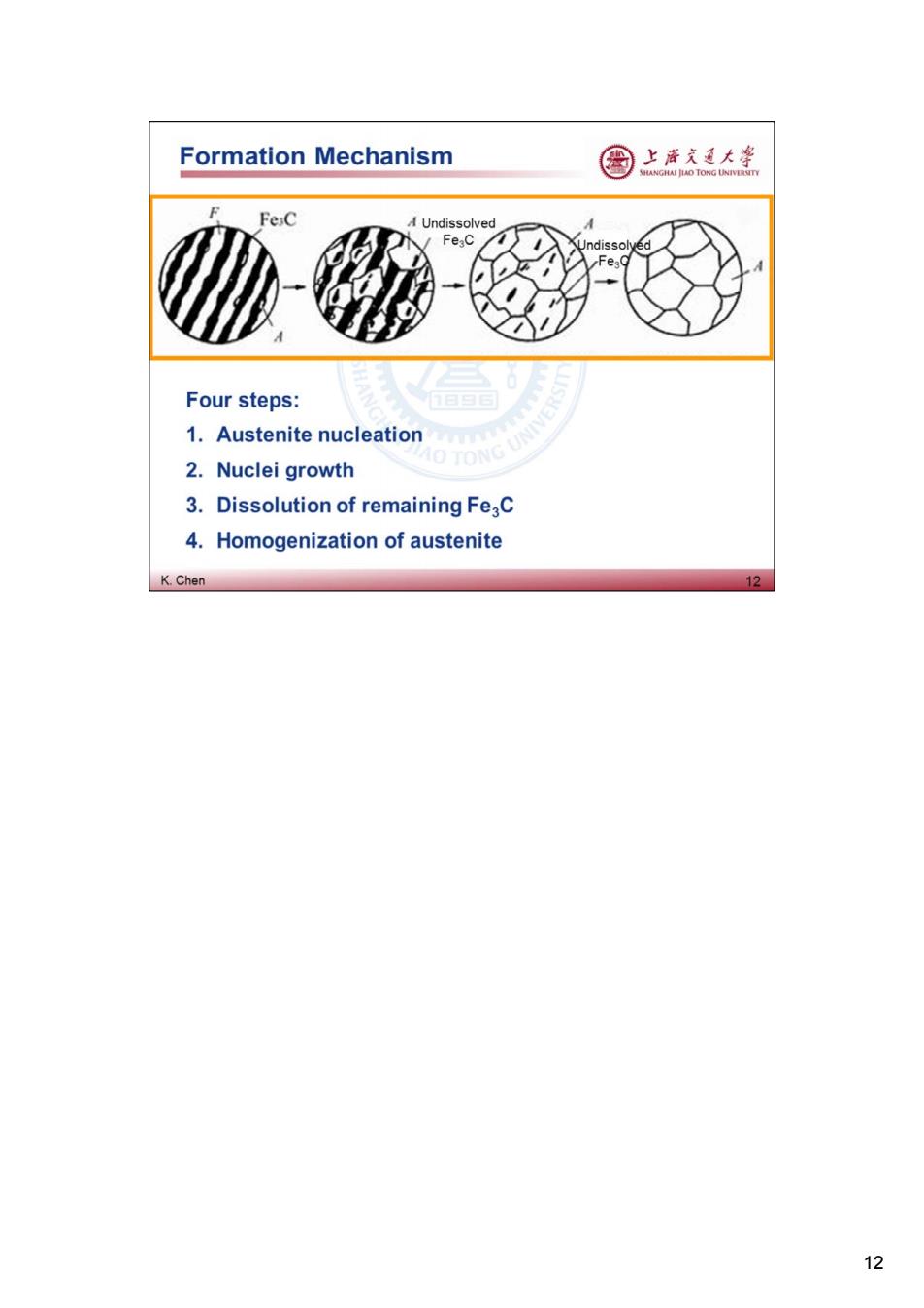
Formation Mechanism 国 上帝充通大学 FeC Undissolved FeaC ed Four steps: 1.Austenite nucleation O TONG UNIVE 2.Nuclei growth 3.Dissolution of remaining Fe2C 4.Homogenization of austenite K.Chen 12 12
12