
上海交通大学 SHANGHAI JIAO TONG UNIVERSITY Chapter 11.Surface and Interface Surface energy surface tension Effect of surface curvature Vapor pressure Solubility of small particles ·Wetting of surfaces ·Gibbs absorption isotherm Review 1
Chapter 11. Surface and Interface Review 1
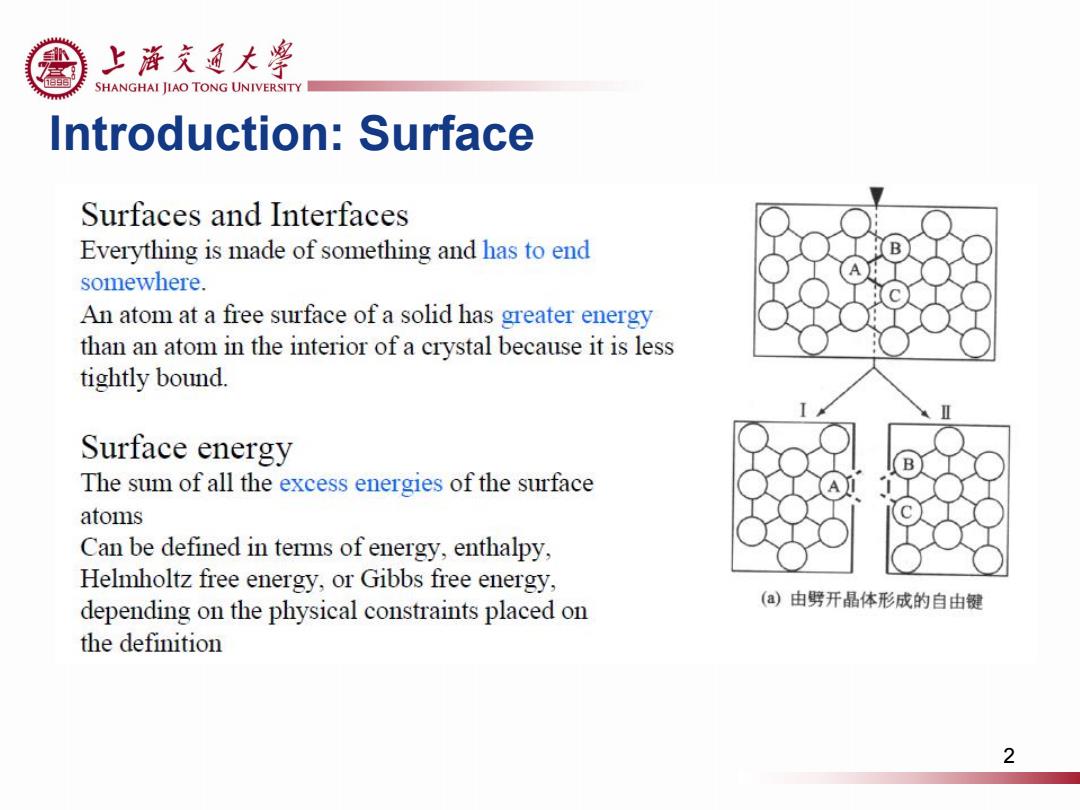
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Introduction:Surface Surfaces and Interfaces Everything is made of something and has to end somewhere. An atom at a free surface of a solid has greater energy than an atom in the interior of a crystal because it is less tightly bound. Surface energy The sum of all the excess energies of the surface atoms Can be defined in terms of energy,enthalpy, Helmholtz free energy,or Gibbs free energy, depending on the physical constraints placed on (a)由劈开晶体形成的自由键 the definition 2
Introduction: Surface 2
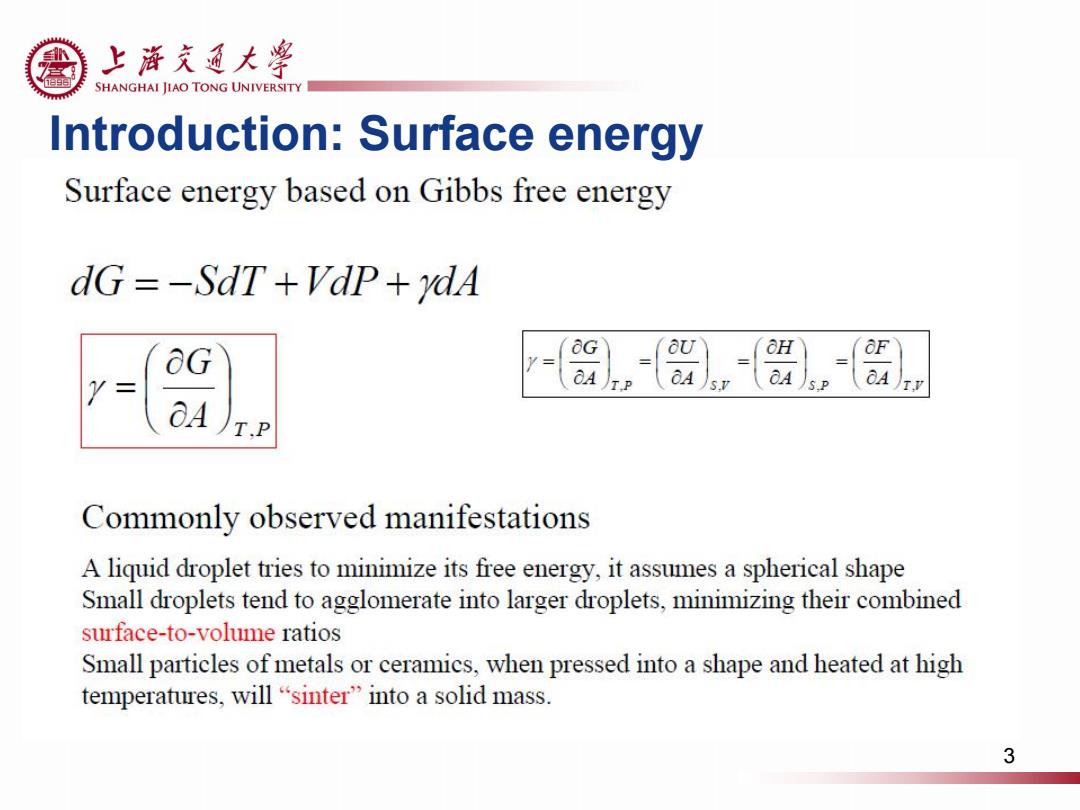
上游充通大粤 e SHANGHAI JIAO TONG UNIVERSITY Introduction:Surface energy Surface energy based on Gibbs free energy dG=-SdT +VaP+ydA OH OF A TP OA A T.P Commonly observed manifestations A liquid droplet tries to minimize its free energy,it assumes a spherical shape Small droplets tend to agglomerate into larger droplets,minimizing their combined surface-to-volume ratios Small particles of metals or ceramics,when pressed into a shape and heated at high temperatures,.will“sinter”into a solid mass. 3
Introduction: Surface energy 3
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY H S U G CHAL HIAO TONG UINIVERSYT F A 4
4

上游充通大粤 SHANGHALILAO TONG UNIVERSITY Bareret Media
5
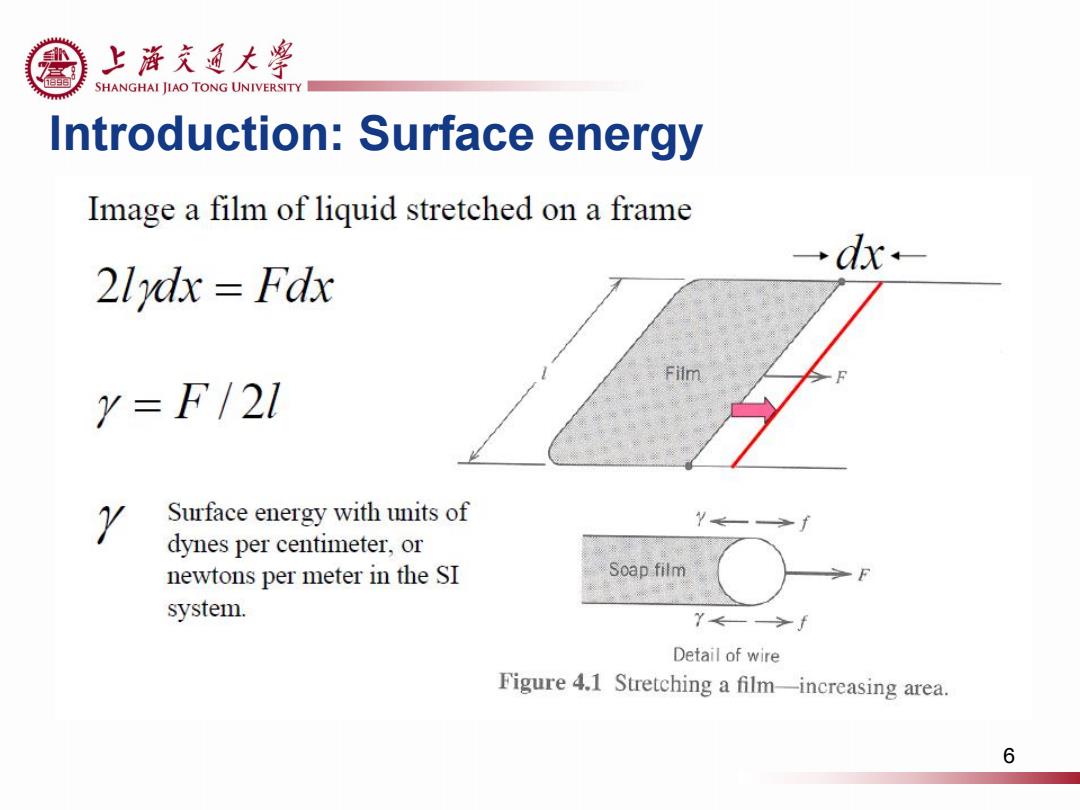
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Introduction:Surface energy Image a film of liquid stretched on a frame →dx- 21ydx Fdx Y=F/21 Y Surface energy with units of dynes per centimeter,or newtons per meter in the SI Soap film system. Y←—>f Detail of wire Figure 4.1 Stretching a film-increasing area. 6
Introduction: Surface energy 6

上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Surface energy surface tension Y Surface energy with units of dynes per centimeter,or newtons per meter in the SI system. [界面张力] [界面能] SI单位制 N(牛顿)·m1=J(焦耳)·m-2 cgs单位制 dyn(达因)·cm-1=erg(尔格)·cm-2 7
Surface energy / surface tension 7

上浒充通大率 SHANGHAI JIAO TONG UNIVERSITY Surface energy surface tension Surface tension or surface energy Liquid Isotropic Surface energy does not change as the surface is stretched Solids Function of the crystallographie plane that is expected The nature of a solid surface changes as the material is deformed 8
Surface energy / surface tension 8
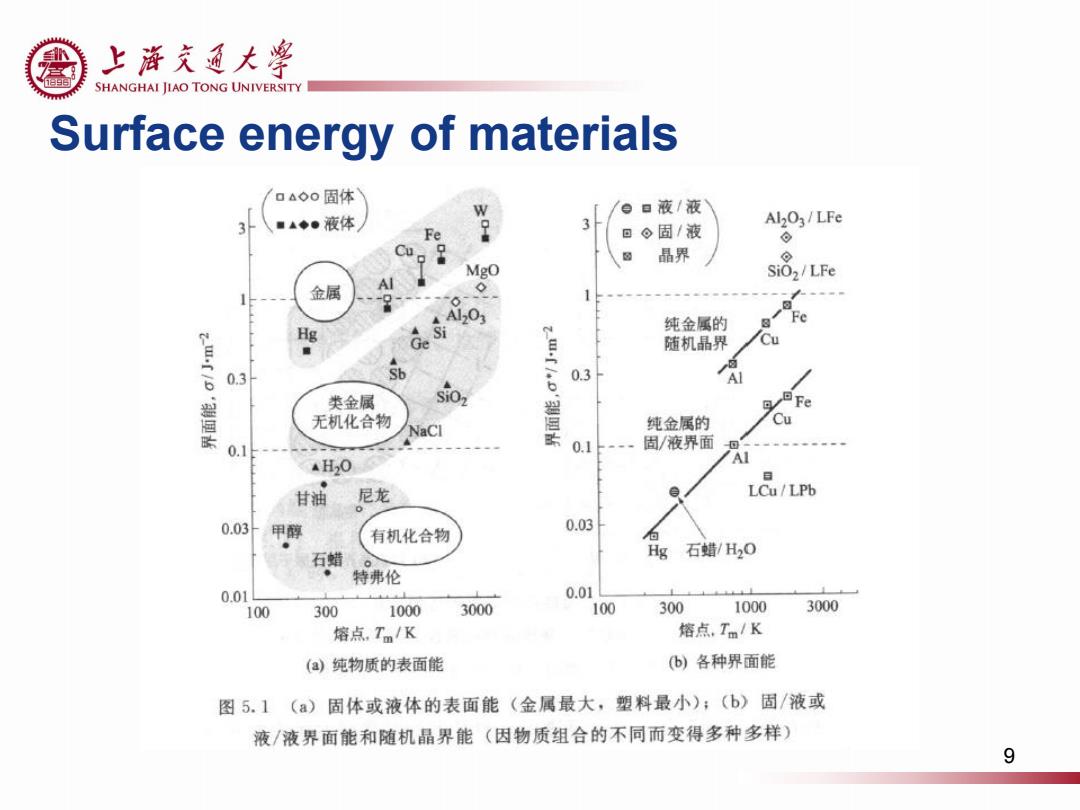
上游充通大¥ e SHANGHAI JIAO TONG UNIVERSITY Surface energy of materials 口△◇0固体 ▣液/液 3 ■▲◆●液体 3 Al2O3/LFe Fe 。固/液 ⊙ 品界 ⊙ Mgo SiOz/LFe 金属 、 A1203 纯金属的 Fe Hg ./o Ge Si 随机晶界 Cu 0.3 0.3 类金属 SiOz 盟 无机化合物 Cu NaCl 纯金属的 0.1 0.1 固/液界面 AH2O Al 日 甘油 尼龙 LCu/LPb 0.03甲醇 0.03 有机化合物 石蜡 Hg 石蜡/H2O 特弗伦 0.01 0.01 100 300 1000 3000 100 300 1000 3000 熔点,Tm/K 熔点,Tm/K (a)纯物质的表面能 (b)各种界面能 图5.1(a) 固体或液体的表面能(金属最大,塑料最小);(b)固/液或 液/液界面能和随机晶界能(因物质组合的不同而变得多种多样) 9
Surface energy of materials 9
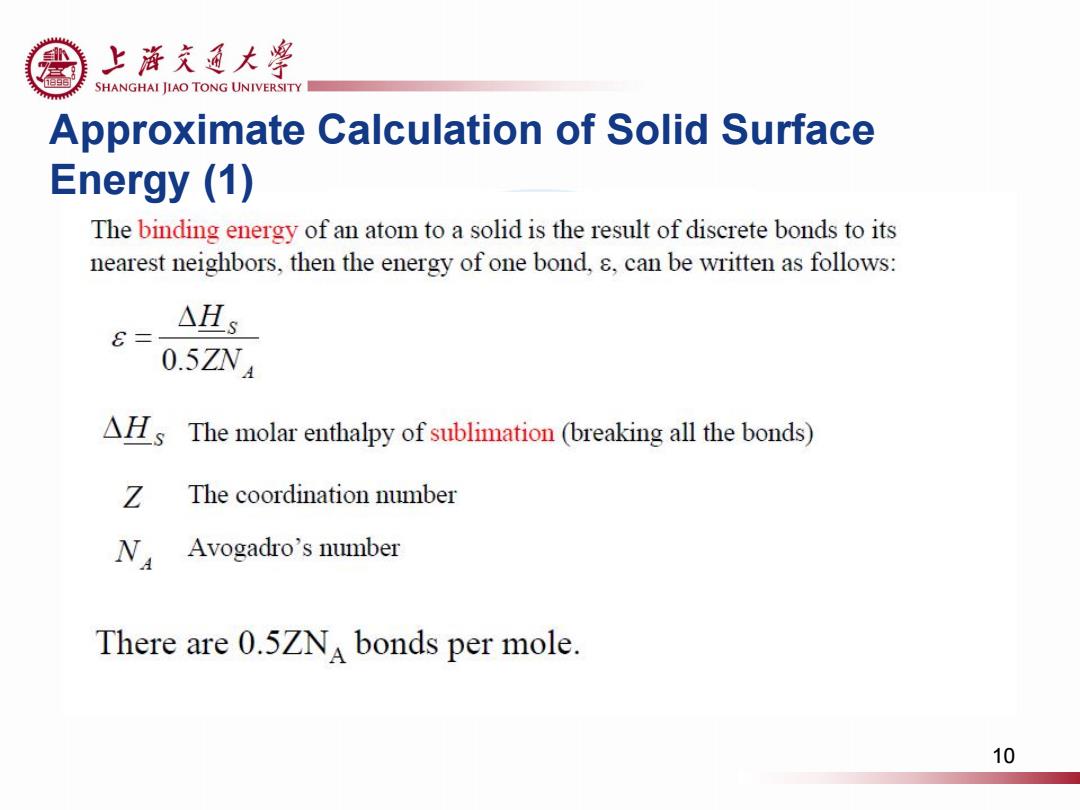
上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY Approximate Calculation of Solid Surface Energy (1) The binding energy of an atom to a solid is the result of discrete bonds to its nearest neighbors,then the energy of one bond,s,can be written as follows: △Hs C= 0.5ZNA AHIs The molar enthalpy of sublimation (breaking all the bonds) Z The coordination number N Avogadro's number There are 0.5ZNA bonds per mole 10
Approximate Calculation of Solid Surface Energy (1) 10