
Contents of Today S.J.T.0. Phase Transformation and Applications Review previous 1th law continuous Adiabatic process:Joule-Thomson expansion绝热过程 Equations of state状态方程 Adiabatic compression or expansion绝热压缩与膨胀 Enthalpies of formation生成焓 Enthalpy changes in chemical reactions焓变与化学反应 Adiabatic temperature change in chemical reactions化学反 应的绝热温度 etc. SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Contents of Today Review previous 1th law continuous Adiabatic process: Joule-Thomson expansion 绝热过程 Equations of state 状态方程 Adiabatic compression or expansion 绝热压缩与膨胀 Enthalpies of formation 生成焓 Enthalpy changes in chemical reactions 焓变与化学反应 Adiabatic temperature change in chemical reactions 化学反 应的绝热温度 etc
Index of nomenclature S.J.T.0. Phase Transformation and Applications Adiabatic绝热 Equations of State状态方程 Non-ideal Gases非理想气体 Enthalpies of Formation生成焓 Exothermic:放热 Endothermic:吸热 Enthalpy of combustion: 燃烧焓 Enthalpy Change in Chemical Reactions化学反应的焓变 AFT:adiabatic flame temperature绝热燃烧温度 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Index of nomenclature Adiabatic绝热 Equations of State状态方程 Non-ideal Gases非理想气体 Enthalpies of Formation生成焓 Exothermic: 放热 Endothermic: 吸热 Enthalpy of combustion:燃烧焓 Enthalpy Change in Chemical Reactions化学反应的焓变 AFT: adiabatic flame temperature绝热燃烧温度 Index of nomenclature

Review Key points S.J.T.0. Phase Transformation and Applications The first law of thermodynamics is simply the principle of conservation of energy:that is,energy can be neither created or destroyed. To derive the practical benefits of knowing this principle, we must construct an accounting system for energy, sometimes called an energy balance. This system must handle flows of energy,such as heat and work,as well as the various forms of energy that matter possesses. To operate this accounting system,we will need to understand a series of basic notions and definitions. SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Review / Key points • The first law of thermodynamics is simply the principle of conservation of energy: that is, energy can be neither created or destroyed. • To derive the practical benefits of knowing this principle, we must construct an accounting system for energy, sometimes called an energy balance. • This system must handle flows of energy, such as heat and work, as well as the various forms of energy that matter possesses. • To operate this accounting system, we will need to understand a series of basic notions and definitions

Review Key points S.J.T.0. Phase Transformation and Applications e System/surroundings.系统/环境 Open/closed/isolated systems敞开/封闭/孤立 ·Thermal equilibrium/steady state平衡稳态 ·Heat/work热功 ·Internal energy内能/热力学能 ·State function状态函数 ·Intensive/extensive properties强度/容量性质 ·Enthalpy焓 ·Heat capacity热容 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Review / Key points • System / surroundings系统/环境 • Open / closed / isolated systems敞开/封闭/孤立 • Thermal equilibrium / steady state平衡稳态 • Heat / work热功 • Internal energy内能/热力学能 • State function状态函数 • Intensive / extensive properties强度/容量性质 • Enthalpy焓 • Heat capacity热容

Review Key points S.J.T.0. Phase Transformation and Applications Systems can be classified as open,close and isolated systems e Oth law 1st law energy conservation ·Heat/work Internal energy enthalpy heat capacity ·State function Mechanical energy Equilibrium /steady state SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Review / Key points • Systems can be classified as open, close and isolated systems • 0th law • 1st law / energy conservation • Heat / work • Internal energy / enthalpy / heat capacity • State function Mechanical energy Equilibrium / steady state
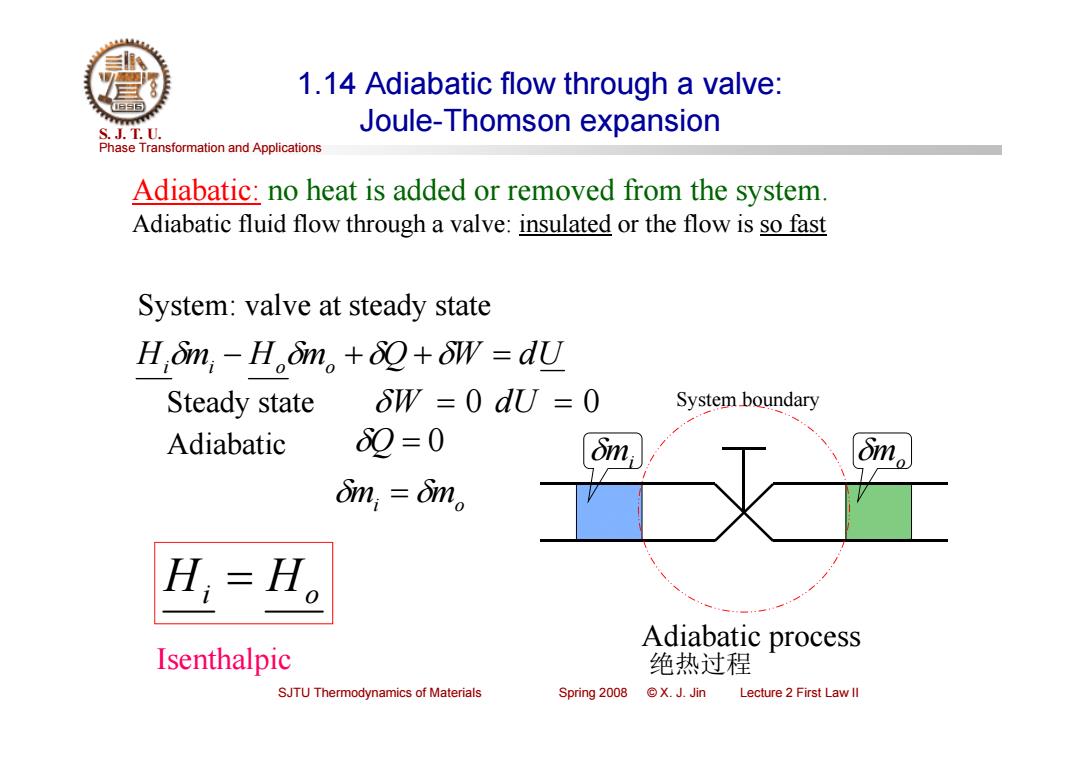
1.14 Adiabatic flow through a valve: S.J.T.0. Joule-Thomson expansion Phase Transformation and Applications Adiabatic:no heat is added or removed from the system. Adiabatic fluid flow through a valve:insulated or the flow is so fast System:valve at steady state H,mn,-H.m。++δW=dU Steady state 8W=0dJ=0 System boundary Adiabatic 89=0 on dim,=6im。 L=H。 Adiabatic process Isenthalpic 绝热过程 SJTU Thermodynamics of Materials Spring 2008 ©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II 1.14 Adiabatic flow through a valve: Joule-Thomson expansion System: valve at steady state Himi Homo Q W dU Adiabatic Q 0 mi mo Hi Ho Isenthalpic System boundary mi mo Adiabatic process 绝热过程 Steady state W 0 dU 0 Adiabatic: no heat is added or removed from the system. Adiabatic fluid flow through a valve: insulated or the flow is so fast
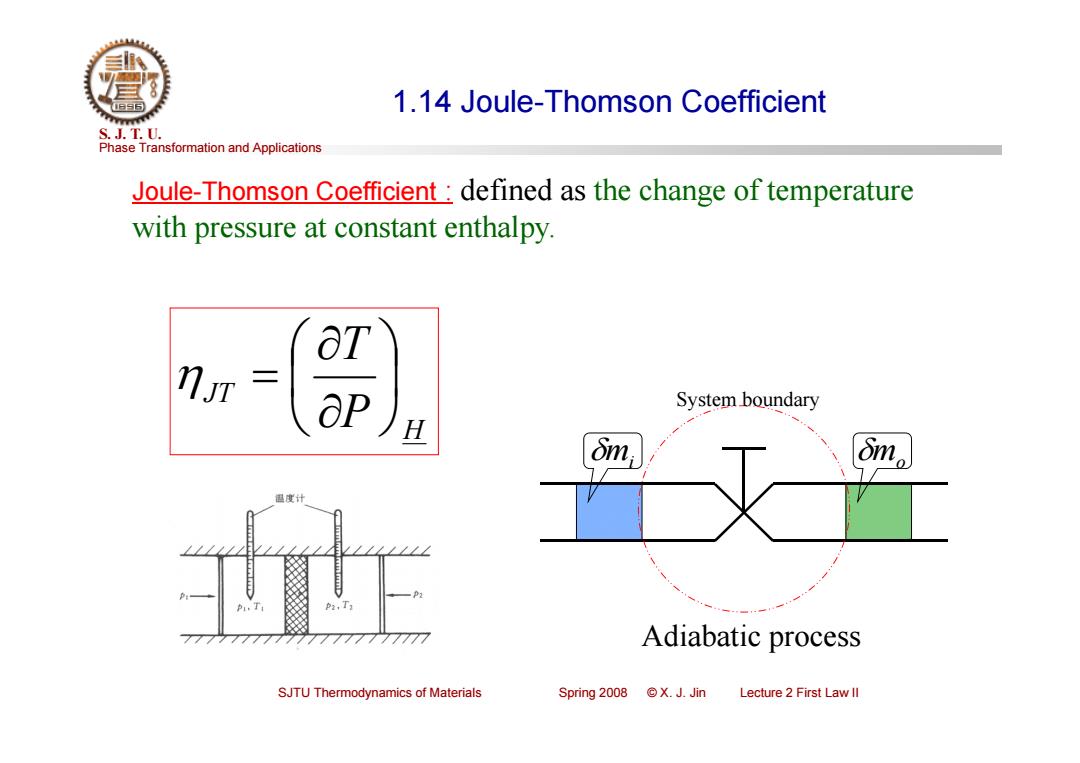
1.14 Joule-Thomson Coefficient S.J.T.0. Phase Transformation and Applications Joule-Thomson Coefficient defined as the change of temperature with pressure at constant enthalpy. ot aP System boundary H dm 国度计 P2,T 7777777777 Adiabatic process SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II 1.14 Joule-Thomson Coefficient Joule-Thomson Coefficient : defined as the change of temperature with pressure at constant enthalpy. System boundary mi mo H JT P T Adiabatic process
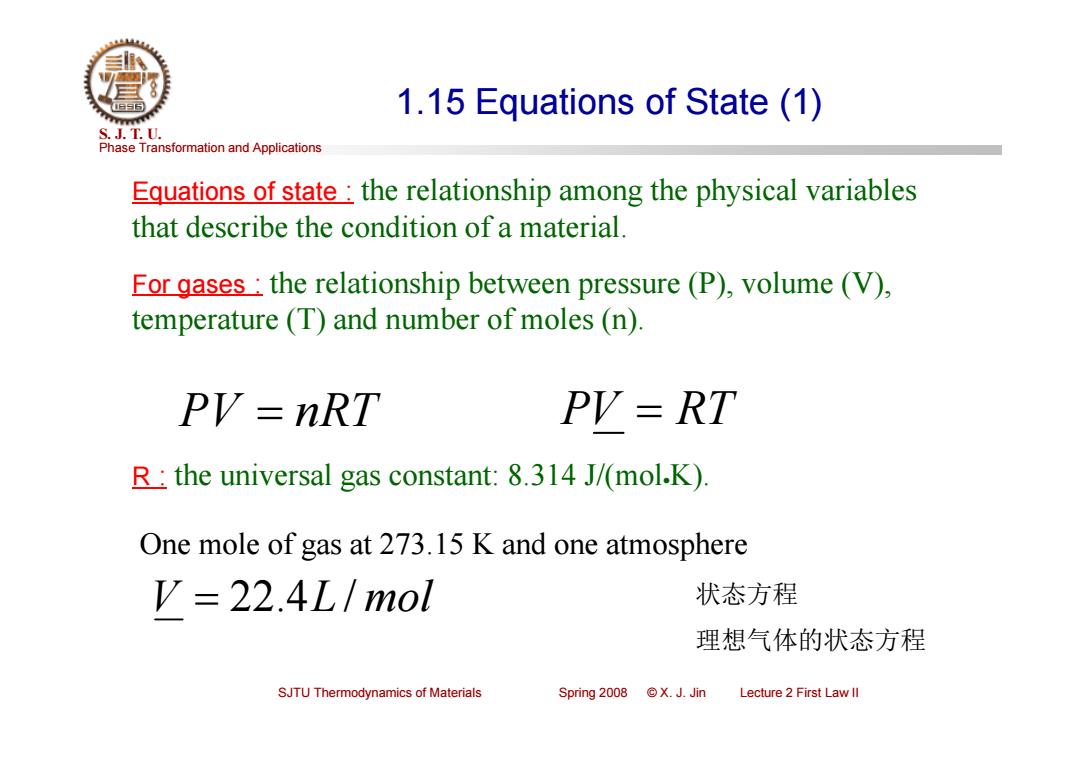
1.15 Equations of State (1) S.J.T.0. Phase Transformation and Applications Equations of state:the relationship among the physical variables that describe the condition of a material. For gases:the relationship between pressure(P),volume (V), temperature (T)and number of moles (n). PV=nRT PV=RT R:the universal gas constant:8.314 J/(mol.K) One mole of gas at 273.15 K and one atmosphere V=22.4L/mol 状态方程 理想气体的状态方程 SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II 1.15 Equations of State (1) Equations of state : the relationship among the physical variables that describe the condition of a material. For gases : the relationship between pressure (P), volume (V), temperature (T) and number of moles (n). PV nRT PV RT R : the universal gas constant: 8.314 J/(molK). V 22.4L / mol One mole of gas at 273.15 K and one atmosphere 状态方程 理想气体的状态方程
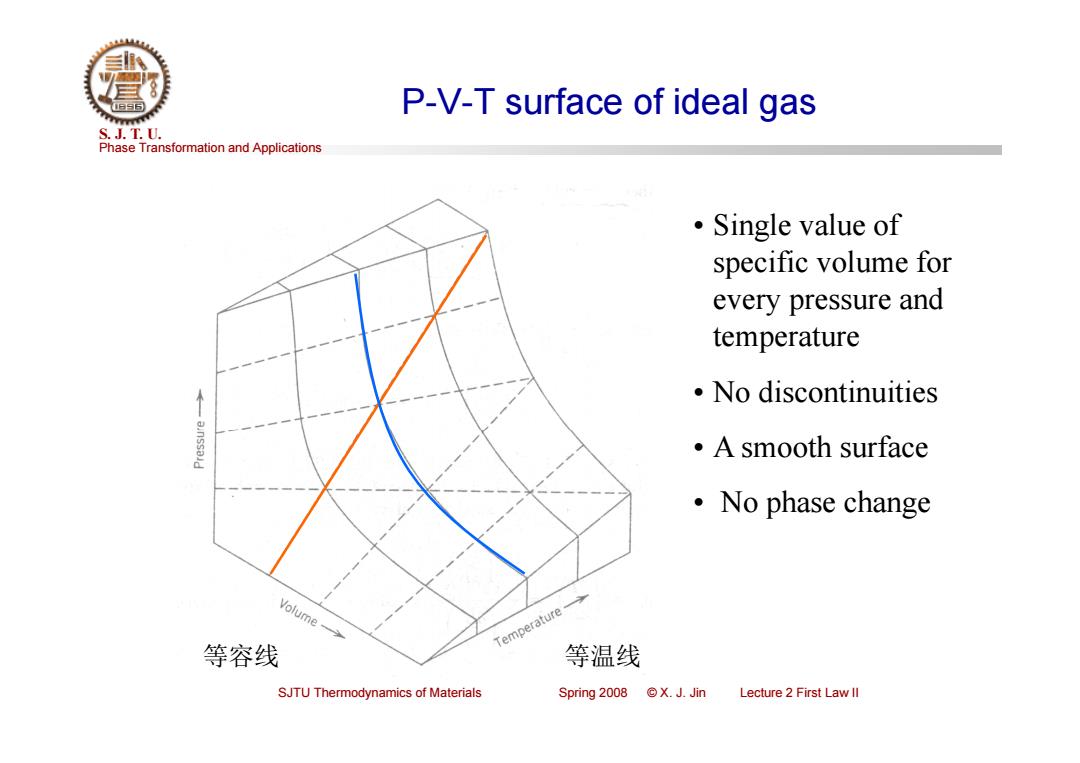
P-V-T surface of ideal gas S.J.T.0. Phase Transformation and Applications ·Single value of specific volume for every pressure and temperature 。No discontinuities ·A smooth surface ·No phase change Volume- Temperature 等容线 等温线 SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II P-V-T surface of ideal gas • Single value of specific volume for every pressure and temperature • No discontinuities • A smooth surface • No phase change 等容线 等温线
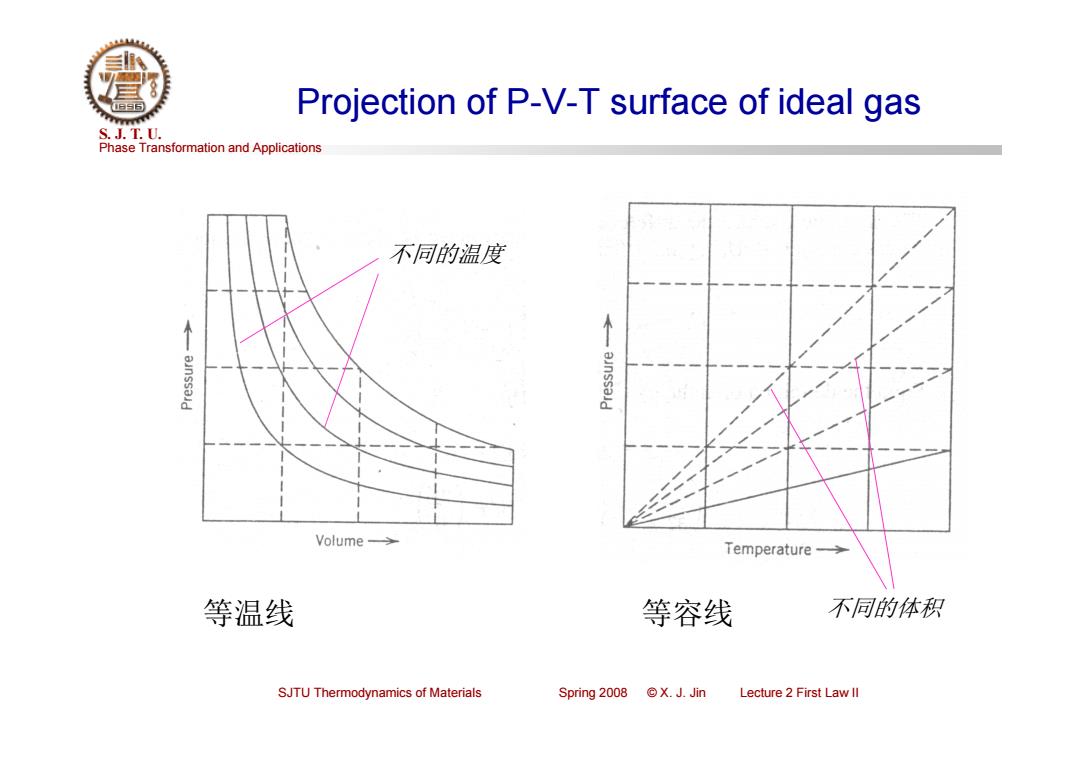
Projection of P-V-T surface of ideal gas S.J.T.0. Phase Transformation and Applications 不同的温度 Volume-> Temperature-> 等温线 等容线 不同的体积 SJTU Thermodynamics of Materials Spring 2008 X.J.Jin Lecture 2 First Law ll
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 2 First Law II Projection of P-V-T surface of ideal gas 等温线 等容线 不同的温度 不同的体积