
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Chapter 8.Phase Rule p/kPa B 水 dp △H (液相) C dTeg Teg△V 22090 dp △fus Hm dr T[n0-'ms】 冰 (固相) 蒸发线 △Ha吧 vapor 101.35 汽 0.611 (气相) 升华线 /10 273.16373.15 647.29 TK 1
Chapter 8. Phase Rule 1
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Nomenclature Phase:相 Component:组元 Degrees of freedom:自由度 Phase rule:相律 Phase diagram:相图 One-component system:单元系 2
Nomenclature 2
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases Stability of a system:How many phases may exist at equilibrium? Phase rule SHA AO TONG UNIVERSITY What is phase? Physically and chemically 3
Phases Physically and chemically Stability of a system: How many phases may exist at equilibrium? Phase rule What is phase? 3

上浒充通大率 19 SHANGHAI JIAO TONG UNIVERSITY Phases(2) in thermodynamics,chemically and physically uniform or homogeneous quantity of matter that can be separated mechanically from a nonhomogeneous mixture and that may consist of a single substance or of a mixture of substances.The three fundamental phases of matter are solid,liquid,and gas (vapour),but others are considered to exist,including crystalline,glassy,amorphous,and plasma phases. > Salt water; >Pure water with two pieces of ice on the surface; > Gold sands and sands; >alloys 4
Phases (2) Ø Salt water; Ø Pure water with two pieces of ice on the surface; Ø Gold sands and sands; Ø alloys 4

上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases Rule Thermodynamic stability of coexisting elements,compounds,and solution Equilibrium structure How many phases 所有多相平衡系统都遵循的普遍规律! 描述平衡系统中相数、组分数以及影响系统状态的独立 可变因素(如温度、压力、组成等)的总数(称为自由 度数)之间的关系。 5
Phases Rule 5

上浒充通大率 SHANGHAI JIAO TONG UNIVERSITY 8.1 Phases 何谓相:物理、化学性质相同的均匀部分 相与相之间有明确界面,越过界面,性质突变 气相,无论多少,1相 •液相,纯、溶液(均匀),1相 多种液体混合,溶解度,1,2,3,… ·固相,纯或原子/分子状态相互混合成固溶体,1相 一般体系中,多一种固体便多一个相 Gas:1 phase Liquid:pure liquid or solution=1 phase Mixture of different liquids:more than 1 phases Solid:pure or atomically/molecularly distributed system,1 phase in general,one more solid=1 more phase 6
8.1 Phases Gas: 1 phase Liquid: pure liquid or solution= 1 phase Mixture of different liquids: more than 1 phases Solid: pure or atomically/molecularly distributed system, 1 phase in general, one more solid= 1 more phase 6

上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY 8.1 Phases(2) 冰、水 冰、盐水溶液 冰、盐水溶液、盐粒 冰、盐水溶液、平衡气相 Ice.water Ice,salt water Ice,salt water,salt particles Ice salt water,equilibrium vapor 7
8.1 Phases (2) Ice, water Ice, salt water Ice, salt water, salt particles Ice ,salt water, equilibrium vapor 7
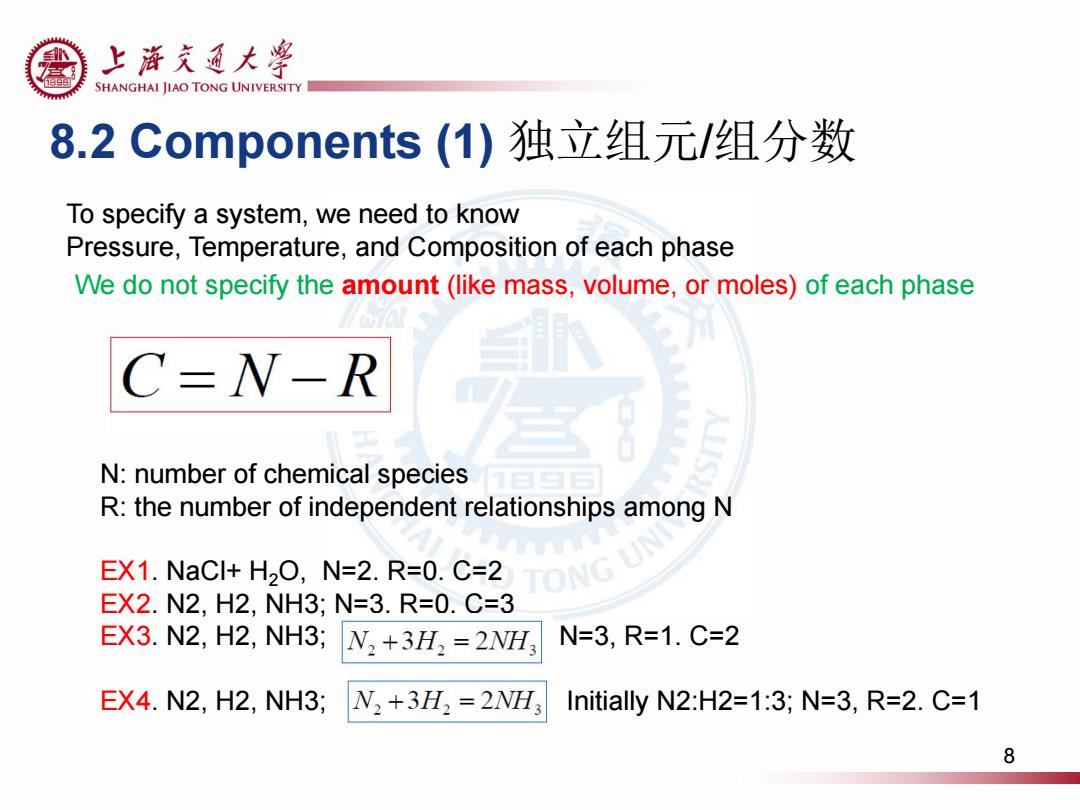
上浒充通大率 SHANGHAI JIAO TONG UNIVERSITY 8.2 Components(1)独立组元/组分数 To specify a system,we need to know Pressure,Temperature,and Composition of each phase We do not specify the amount(like mass,volume,or moles)of each phase C-N-R N:number of chemical species R:the number of independent relationships among N EX1.NaCl+H2O,N=2.R=0.C=2 TONG EX2.N2,H2,NH3;N=3.R=0.C=3 EX3.N2,H2,NH3; N,+3H,=2NH N=3,R=1.C=2 EX4.N2,H2,NH3; N2+3H2=2WH3 Initially N2:H2=1:3;N=3,R=2.C=1 8
8.2 Components (1) 独立组元/组分数 To specify a system, we need to know Pressure, Temperature, and Composition of each phase N: number of chemical species R: the number of independent relationships among N EX1. NaCl+ H2O, N=2. R=0. C=2 EX2. N2, H2, NH3; N=3, R=0. C=3 EX3. N2, H2, NH3; N=3, R=1. C=2 EX4. N2, H2, NH3; , Initially N2:H2=1:3; N=3, R=2. C=1 We do not specify the amount (like mass, volume, or moles) of each phase 8
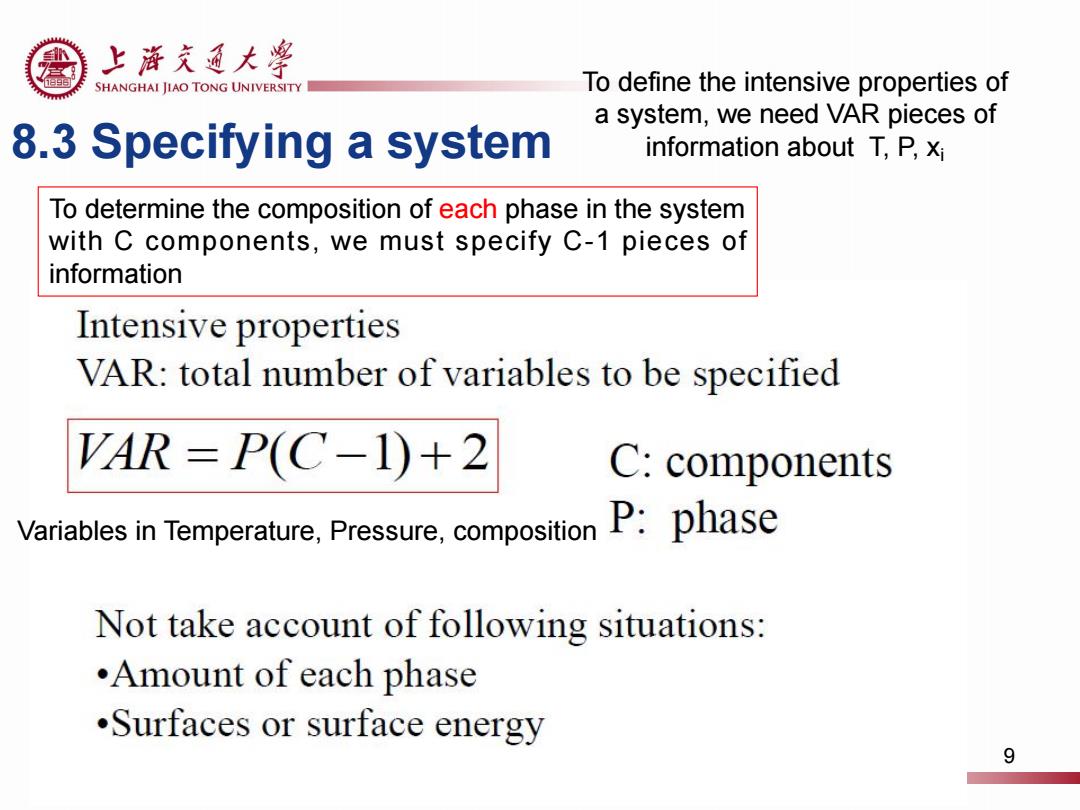
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY To define the intensive properties of a system,we need VAR pieces of 8.3 Specifying a system information about T,P,xi To determine the composition of each phase in the system with C components,we must specify C-1 pieces of information Intensive properties VAR:total number of variables to be specified VAR=P(C-1)+2 C:components Variables in Temperature,Pressure,composition P:phase Not take account of following situations: •Amount of each phase .Surfaces or surface energy 9
8.3 Specifying a system To determine the composition of each phase in the system with C components, we must specify C-1 pieces of information Variables in Temperature, Pressure, composition To define the intensive properties of a system, we need VAR pieces of information about T, P, xi 9
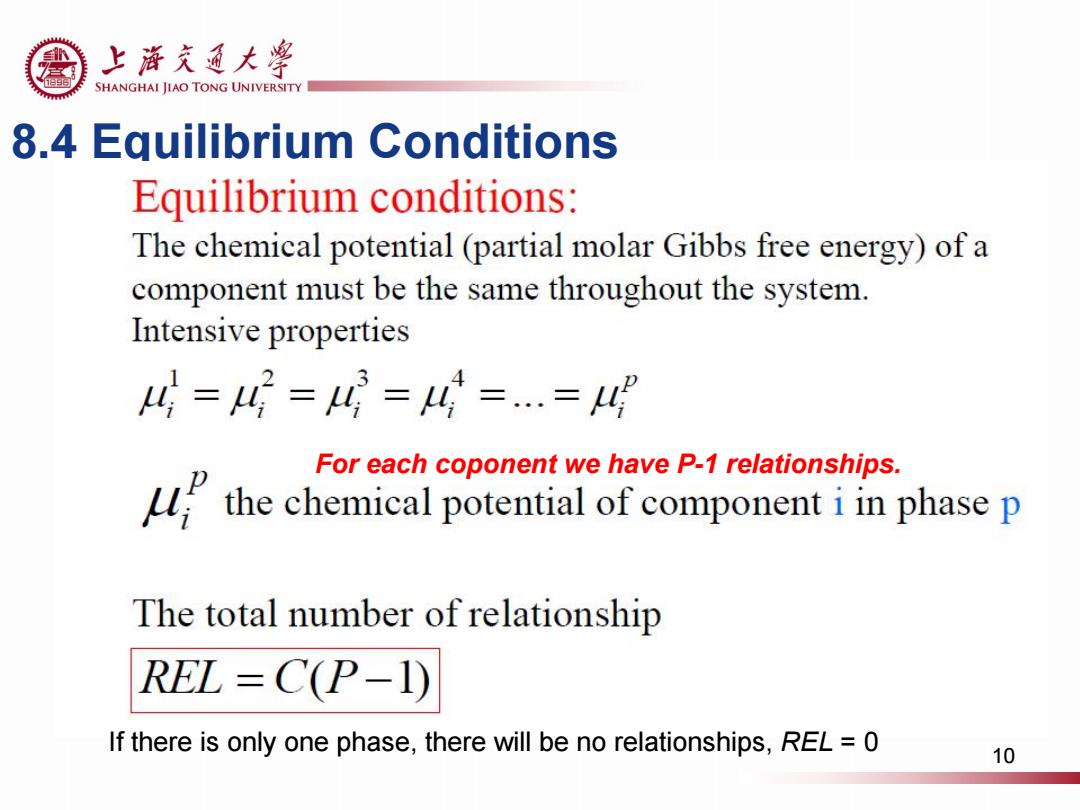
上浒充通大粤 19 SHANGHAI JIAO TONG UNIVERSITY 8.4 Equilibrium Conditions Equilibrium conditions: The chemical potential(partial molar Gibbs free energy)of a component must be the same throughout the system. Intensive properties 4=4=4=4=.=4 For each coponent we have P-1 relationships. the chemical potential of component phase p The total number of relationship REL =C(P-1) If there is only one phase,there will be no relationships,REL =0 10
8.4 Equilibrium Conditions If there is only one phase, there will be no relationships, REL = 0 For each coponent we have P-1 relationships. 10