
8/18/2016 What happens when milk sours or a tree Microbes rots? Microbes are conducting chemical reactions Bacteria decompose the wood Bacteria produce lactic acid that sours the milk Invisible Invaders Amazing Allies Knowledge of chemistry is vital to understand: what roles microorganisms play in nature how microbes cause disease how to develop diagnostic methods to detect disease how the body wards off infection Chapter 2 how antibiotics and vaccine are produced Chemical Principles changes that occur in microbes changes that microbes make in our environment Atoms:Basic Building Blocks of all Types Structure of Atoms Matter All atoms have a nucleus and Matter-all materials that occupy space and electrons that move around the e have mass nucleus ●Atoms are composed of Matter is composed of atoms protons-positively(+)charged particles Atom-simplest component of pure neutrons-neutral uncharged particles substance that is not divisible into simpler electrons-negatively(-)charged particles substances Charge is a property of particles that produce a repulsive or Atoms interact with each other to form attractive force molecules Chemical element-all atoms with the same number of nave the e way chemically Chemistry is the science of the interaction between atoms and molecules If the outer most shell is partially filled,the atom is unstable and can react with other atoms
8/18/2016 1 Invisible Invaders Amazing Allies Chapter 2 Chemical Principles What happens when milk sours or a tree rots? Microbes are conducting chemical reactions Bacteria decompose the wood Bacteria produce lactic acid that sours the milk Knowledge of chemistry is vital to understand: what roles microorganisms play in nature how microbes cause disease how to develop diagnostic methods to detect disease how the body wards off infection how antibiotics and vaccine are produced changes that occur in microbes changes that microbes make in our environment Matter - all materials that occupy space and have mass Matter is composed of atoms Atom – simplest component of pure substance that is not divisible into simpler substances Atoms interact with each other to form molecules Chemistry is the science of the interaction between atoms and molecules Atoms: Basic Building Blocks of all Types Matter All atoms have a nucleus and electrons that move around the nucleus Atoms are composed of protons – positively (+) charged particles neutrons – neutral uncharged particles electrons - negatively (-) charged particles Charge is a property of particles that produce a repulsive or attractive force Chemical element – all atoms with the same number of protons that behave the same way chemically Outermost electron shell can give up or accept electrons from other atoms If the outer most shell is partially filled, the atom is unstable and can react with other atoms. Structure of Atoms

8/18/2016 Chemical Bonds Three Types of Chemical Bonds he compironfe me Atoms are electrically neutral when positive ination of two or more atoms-the same charges equal negative charges eg3a8r-earatgtg2otleast2or An ion is a negatively or positively charged atom resulting from the loss or gain of electrons cmpounds are molecules but not all molecules ae 1.Covalent bonds-electrons are shared among atoms Chemical bonds-when 2 or more atoms share,donate molecules and compounds: polar covalent bonds-unequal sharing attractive forces are formed nonpolar covalent bonds-equal sharing 3 types of chemical bonds covalent 回●)→⊙●) ionic hydrogen a0 回可 Three Types of Chemical Bonds Three Types of Chemical Reactions 2.Ionic bonds-electrons are transferred to Synthesis Reactions (anabolism) one atom forming positively charged cations Two or more molecules combine to form a larger molecule and negatively charged anions-formed by A+B -AB attraction sugar molecules to form starch 3.Hydrogen bonds-weak electrostatic bonds amino acids to form protein between hydrogen and other atoms,O and N Decomposition Reactions (catabolism) Split larger molecules into smaller parts AB-A+B bonds are broken breakdown of sucrose into fructose and glucose Exchange Reactions Part synthesis and part decomposition Bonds are broken between two molecules and new bonds are formed to synthesize new molecules AB +CD ->AD BC 2
8/18/2016 2 Molecule – distinct chemical substance that results from the combination of two or more atoms - the same or different atoms - oxygen Compounds – a molecule that contains at least 2 or more different atoms, such as water All compounds are molecules but not all molecules are compounds Chemical bonds – when 2 or more atoms share, donate or accept electrons to form molecules and compounds; attractive forces are formed 3 types of chemical bonds covalent ionic hydrogen Chemical Bonds Atoms are electrically neutral when positive charges equal negative charges An ion is a negatively or positively charged atom resulting from the loss or gain of electrons 1. Covalent bonds – electrons are shared among atoms polar covalent bonds– unequal sharing nonpolar covalent bonds– equal sharing Three Types of Chemical Bonds Covalent bond 2. Ionic bonds – electrons are transferred to one atom forming positively charged cations and negatively charged anions – formed by attraction 3. Hydrogen bonds – weak electrostatic bonds between hydrogen and other atoms, O and N Three Types of Chemical Bonds Ionic bond Synthesis Reactions (anabolism) Two or more molecules combine to form a larger molecule A + B AB sugar molecules to form starch amino acids to form protein Decomposition Reactions (catabolism) Split larger molecules into smaller parts AB A + B bonds are broken breakdown of sucrose into fructose and glucose Exchange Reactions Part synthesis and part decomposition Bonds are broken between two molecules and new bonds are formed to synthesize new molecules AB + CD AD + BC Three Types of Chemical Reactions

8/18/2016 Important Biological Molecules Inorganic Compounds Inorganic Compounds Small and structurally simple Learning Objectives Typically lack carbon Examples:water,oxygen,carbon dioxide,salts, List several properties of water that are acids,and bases important to living systems Define acid,base,salt,and pH Organic Compounds Always contain carbon and hydroger Examples:structurally complex polysaccharides, proteins,and nucleic acids(macromolecules) Water Water is a Good Medium for Living Cells All living organisms require a variety of Exists mostly as liguid on Earth inorganic compounds for growth,repair, -strong attraction for other water molecules maintenance,and reproduction requires heat to form vapor == Water is vital and makes up on average 65%- Good solvent(dissolving medium)due to 75%of every cell polarity For microorganisms,outside the cell nutrients polar substances dissociate forming solutes are dissolved in water,which aids their passage into the cell Key reactant in the digestive process and in the synthesis of organic compounds in cells Water is a polar molecule since it -H'and O participate in chemical reactions has a slight negative and a Maintains a constant temperatur slight positive charge H a good temperature buffer for living cells Polar molecule Due to the H-bond of water molecules
8/18/2016 3 Inorganic Compounds Small and structurally simple Typically lack carbon Examples: water, oxygen, carbon dioxide, salts, acids, and bases Organic Compounds Always contain carbon and hydrogen Examples: structurally complex polysaccharides, proteins, and nucleic acids (macromolecules) Important Biological Molecules Learning Objectives List several properties of water that are important to living systems Define acid, base, salt, and pH Inorganic Compounds All living organisms require a variety of inorganic compounds for growth, repair, maintenance, and reproduction Water is vital and makes up on average 65%- 75% of every cell For microorganisms, outside the cell nutrients are dissolved in water, which aids their passage into the cell Water is a polar molecule since it has a slight negative and a slight positive charge Water Polar molecule Water is a Good Medium for Living Cells Exists mostly as liquid on Earth - strong attraction for other water molecules - requires heat to form vapor Good solvent (dissolving medium) due to polarity - polar substances dissociate forming solutes Key reactant in the digestive process and in the synthesis of organic compounds in cells - H+ and O- participate in chemical reactions Maintains a constant temperature providing a good temperature buffer for living cells - Due to the H-bond of water molecules Water acts as a solvent for sodium chloride

8/18/2016 Acids Acids,Bases,and Salts An acid is a substance that When inorganic salts are dissolved in dissociates into one or more water,they undergo ionization or hydrogen(H)ion dissociation. They break apart into ions. An ion is a negatively or positively HCI→H+C charged atom. (a)Acid An acid can be defined as a In water. proton(H)donor hydrochloric acid (HCI)dissociates into hydrogen and chloride ions Bases Salts A base is a substance that ●A salt is a substance that dissociates into NaOH->Na'+OH- cations and anions, neither of which is H'or oH-. A hydroxide ion(OH-)car combine with protons and is a proton (H)acceptor In water. .sodium NaCl→Na*+CH (e)Sait hydroxide (NaOH) dissociates into In water,sodium chloride hydroxide and (NaCl)dissociates into sodium ions chloride and sodium ions 4
8/18/2016 4 Acids, Bases, and Salts • When inorganic salts are dissolved in water, they undergo ionization or dissociation. • They break apart into ions. • An ion is a negatively or positively charged atom. An acid is a substance that dissociates into one or more hydrogen (H+) ion HCl H+ + Cl An acid can be defined as a proton (H+) donor Acids In water, hydrochloric acid (HCl) dissociates into hydrogen and chloride ions Bases In water, sodium hydroxide (NaOH) dissociates into hydroxide and sodium ions A base is a substance that dissociates into one or more hydroxide ion (OH) NaOH Na+ + OH A hydroxide ion (OH-) can combine with protons and is a proton (H+) acceptor A salt is a substance that dissociates into cations and anions, neither of which is H+ or OH. NaCl Na+ + Cl Salts In water, sodium chloride (NaCl) dissociates into chloride and sodium ions

8/18/2016 Acid-Base Balance:the Concept of pH The amount of Hin a solution is expressed as pH(potential of hydrogen) Increasing [H+],increases acidity Increasing [OH-]increases alkalinity Most organisms grow best between pH 6.5-8.5 H'1IOM Too high or too low pH.enzymes change in shape cannot promote chemical reactions in a cell Chemical reactions in a cell are sensitive to pH Most biochemical processes in a cell involve OH- and Hions Organic Compounds Structure and Chemistry Learning Objectives Inorganic compounds,excluding water, Distinguish organic and inorganic compounds constitute 1%-1.5%of cells Identify the building blocks of carbohydrates Organic molecules,whose carbon atoms Differentiate simple lipids,complex lipids, can combine in many ways with carbon and steroids atoms of other elements,are complex and Identify the building blocks and structure of can perform complex biological functions proteins Small organic molecules can combine into large macromolecules Identify the building blocks of nucleic acids Describe the role of ATP in cellular reactions 5
8/18/2016 5 Acid-Base Balance: the Concept of pH The amount of H+ in a solution is expressed as pH (potential of hydrogen) Increasing [H+], increases acidity Increasing [OH] increases alkalinity Most organisms grow best between pH 6.5 - 8.5 Too high or too low pH, enzymes change in shape cannot promote chemical reactions in a cell Chemical reactions in a cell are sensitive to pH Most biochemical processes in a cell involve OH and H+ ions Learning Objectives Distinguish organic and inorganic compounds Identify the building blocks of carbohydrates Differentiate simple lipids, complex lipids, and steroids Identify the building blocks and structure of proteins Identify the building blocks of nucleic acids Describe the role of ATP in cellular reactions Organic Compounds Structure and Chemistry Inorganic compounds, excluding water, constitute 1% - 1.5% of cells Organic molecules, whose carbon atoms can combine in many ways with carbon atoms of other elements, are complex and can perform complex biological functions Small organic molecules can combine into large macromolecules

8/18/2016 Macromolecules are polymers consisting of Carbohydrates many small repeating molecules The smaller molecules are called monomers Carbohydrates are a large diverse group of Monomers join by dehydration synthesis or organic compounds that include sugarsand condensation reactions starches The reaction involves the elimination of a Perform a number of functions hydrogen from one monomer and a -building blocks for nucleic acids hydroxyl from the other -synthesis of amino acids needed for proteins -fats needed for membranes cell wall synthesis food reserves -source of energy to fuel activities of the cell Consist of C,H,and O with the formula(CH2O)n Carbohydrates:Three Major Groups Lipids Morosaccharides are simple sugars with 3-7 carbon atoms Glucose-main energy-supplying molecule in the cell Essential for structure and function of membrane ●Consist of C,H,andO ewlare comedof isiddpoen Are nonpolar(do not have a positive and negative end like water) Polysaccharides consist of tens or hundreds of Are insoluble in water but are soluble in nonpolar solvents (ether) Function in energy storage,membrane structure, and some as hormones(steroids) produce cellulases Three types of lipids Simple lipids(fats)or triglycerides Complex lipids such as phospholipids Steroids 6
8/18/2016 6 Macromolecules are polymers consisting of many small repeating molecules The smaller molecules are called monomers Monomers join by dehydration synthesis or condensation reactions The reaction involves the elimination of a hydrogen from one monomer and a hydroxyl from the other Carbohydrates Carbohydrates are a large diverse group of organic compounds that include sugars and starches Perform a number of functions - building blocks for nucleic acids - synthesis of amino acids needed for proteins - fats needed for membranes - cell wall synthesis - food reserves - source of energy to fuel activities of the cell Consist of C, H, and O with the formula (CH2O)n Carbohydrates: Three Major Groups Monosaccharides are simple sugars with 3-7 carbon atoms Glucose - main energy-supplying molecule in the cell Disaccharides are formed when two monosaccharides are joined in a dehydration synthesis Bacterial cell walls are composed of disaccharide and protein Polysaccharides consist of tens or hundreds of monosaccharides joined through dehydration synthesis and are usually not soluble in water Cellulose is a glucose polymer, most abundant carbohydrate on Earth, and is only broken down by bacteria or fungi that produce cellulases Lipids Essential for structure and function of membranes Consist of C, H, and O Are nonpolar (do not have a positive and negative end like water) Are insoluble in water but are soluble in nonpolar solvents (ether) Function in energy storage, membrane structure, and some as hormones (steroids) Three types of lipids Simple lipids (fats) or triglycerides Complex lipids such as phospholipids Steroids
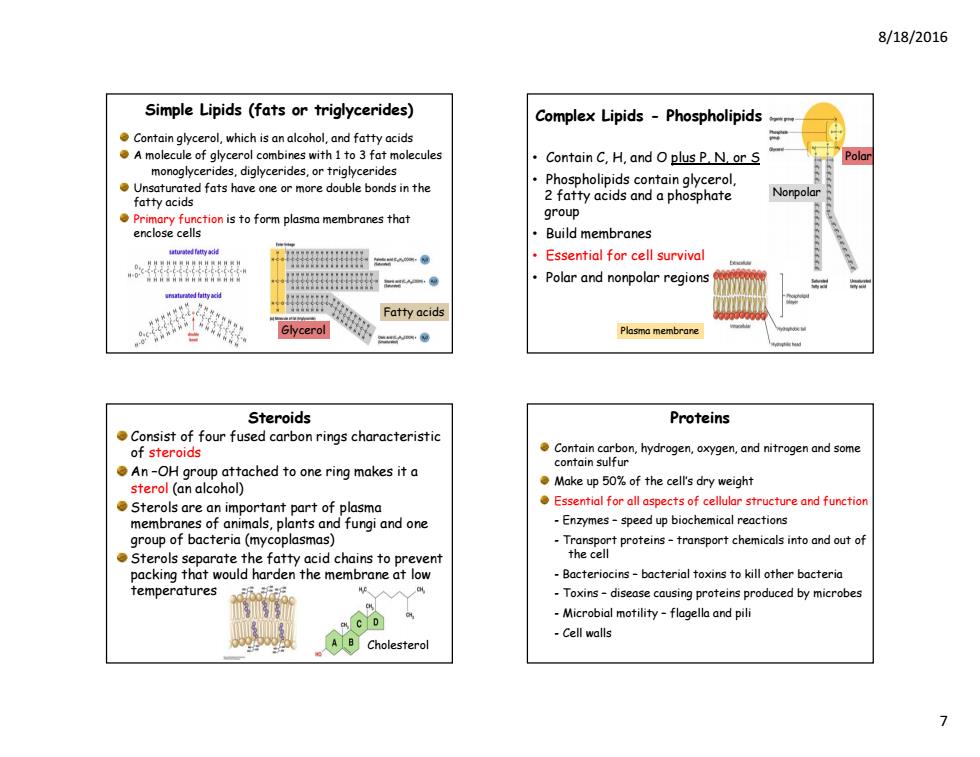
8/18/2016 Simple Lipids (fats or triglycerides) Complex Lipids -Phospholipids Contain glycerol,which is an alcohol,and fatty acids A molecule of glycerol combines with 1 to 3 fat molecules Contain C,H,and O plus P N.or S monoglycerides,diglycerides,or triglycerides Unsaturated fats have one or more double bonds in the Phospholipids contain glycerol, fatty acids 2 fatty acids and a phosphate Primary function is to form plasma membranes that group enclose cells Build membranes Essential for cell survival Polar and nonpolar regions Fatty acids Glycerol Plasma membrane Steroids Proteins Consist of four fused carbon rings characteristic of steroids Contain carbon,hydrogen,oxygen,and nitrogen and some An-OH group attached to one ring makes it a contain sulfur sterol (an alcohol) Make up 50%of the cell's dry weight Sterols are an important part of plasma Essential for all aspects of cellular structure and functior membranes of animals,plants and fungi and one -Enzymes-speed up biochemical reactions group of bacteria (mycoplasmas) Transport proteins-transport chemicals into and out of Sterols separate the fatty acid chains to prevent the cell packing that would harden the membrane at low Bacteriocins-bacterial toxins to kill other bacteria temperatures -Toxins-disease causing proteins produced by microbes C D Microbial motility-flagella and pili ACholesterol -Cell walls
8/18/2016 7 Contain glycerol, which is an alcohol, and fatty acids A molecule of glycerol combines with 1 to 3 fat molecules monoglycerides, diglycerides, or triglycerides Unsaturated fats have one or more double bonds in the fatty acids Primary function is to form plasma membranes that enclose cells Simple Lipids (fats or triglycerides) Glycerol Fatty acids • Contain C, H, and O plus P, N, or S • Phospholipids contain glycerol, 2 fatty acids and a phosphate group • Build membranes • Essential for cell survival • Polar and nonpolar regions Complex Lipids - Phospholipids Nonpolar Polar Plasma membrane Steroids Consist of four fused carbon rings characteristic of steroids An –OH group attached to one ring makes it a sterol (an alcohol) Sterols are an important part of plasma membranes of animals, plants and fungi and one group of bacteria (mycoplasmas) Sterols separate the fatty acid chains to prevent packing that would harden the membrane at low temperatures Cholesterol Proteins Contain carbon, hydrogen, oxygen, and nitrogen and some contain sulfur Make up 50% of the cell’s dry weight Essential for all aspects of cellular structure and function - Enzymes – speed up biochemical reactions - Transport proteins – transport chemicals into and out of the cell - Bacteriocins – bacterial toxins to kill other bacteria - Toxins – disease causing proteins produced by microbes - Microbial motility – flagella and pili - Cell walls
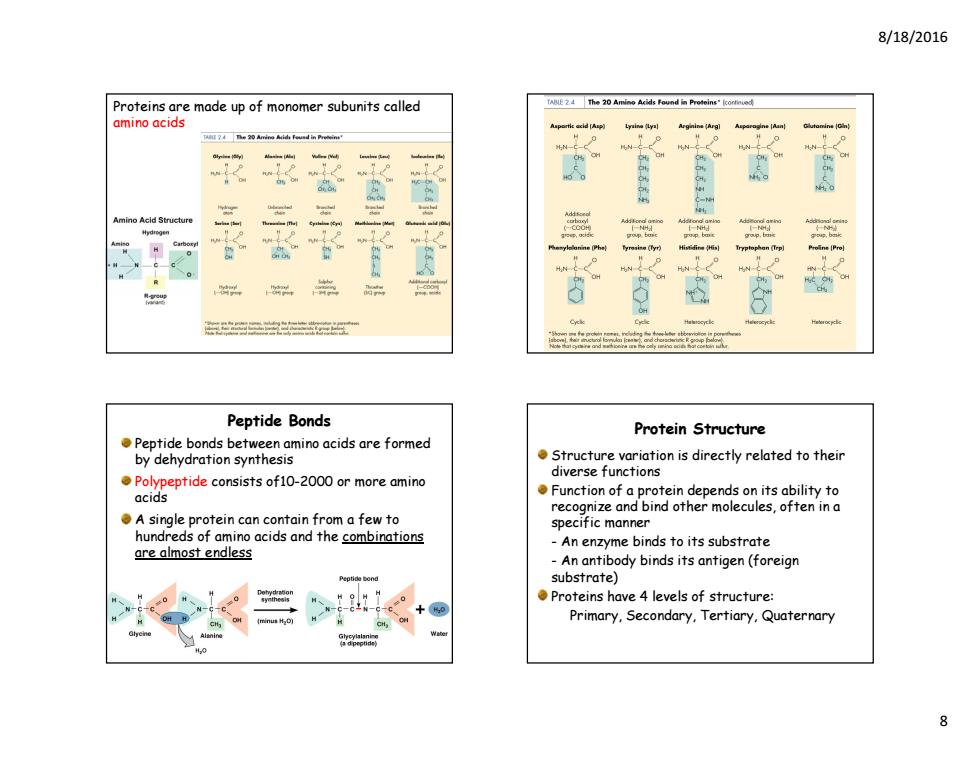
8/18/2016 Proteins are made up of monomer subunits called amino acids 求家 童 督 Amino Acid Structur 总 Peptide Bonds Protein Structure Peptide bonds between amino acids are formed by dehydration synthesis Structure variation is directly related to their diverse functions Polypeptide consists of10-2000 or more amino acids Function of a protein depends on its ability to recognize and bind other molecules,often in a A single protein can contain from a few to specific manner hundreds of amino acids and the combinations An enzyme binds to its substrate are almost endless -An antibody binds its antigen(foreign substrate) Proteins have 4 levels of structure: Primary,Secondary,Tertiary,Quaternary 8
8/18/2016 8 Proteins are made up of monomer subunits called amino acids Peptide bonds between amino acids are formed by dehydration synthesis Polypeptide consists of10-2000 or more amino acids A single protein can contain from a few to hundreds of amino acids and the combinations are almost endless Peptide Bonds Structure variation is directly related to their diverse functions Function of a protein depends on its ability to recognize and bind other molecules, often in a specific manner - An enzyme binds to its substrate - An antibody binds its antigen (foreign substrate) Proteins have 4 levels of structure: Primary, Secondary, Tertiary, Quaternary Protein Structure
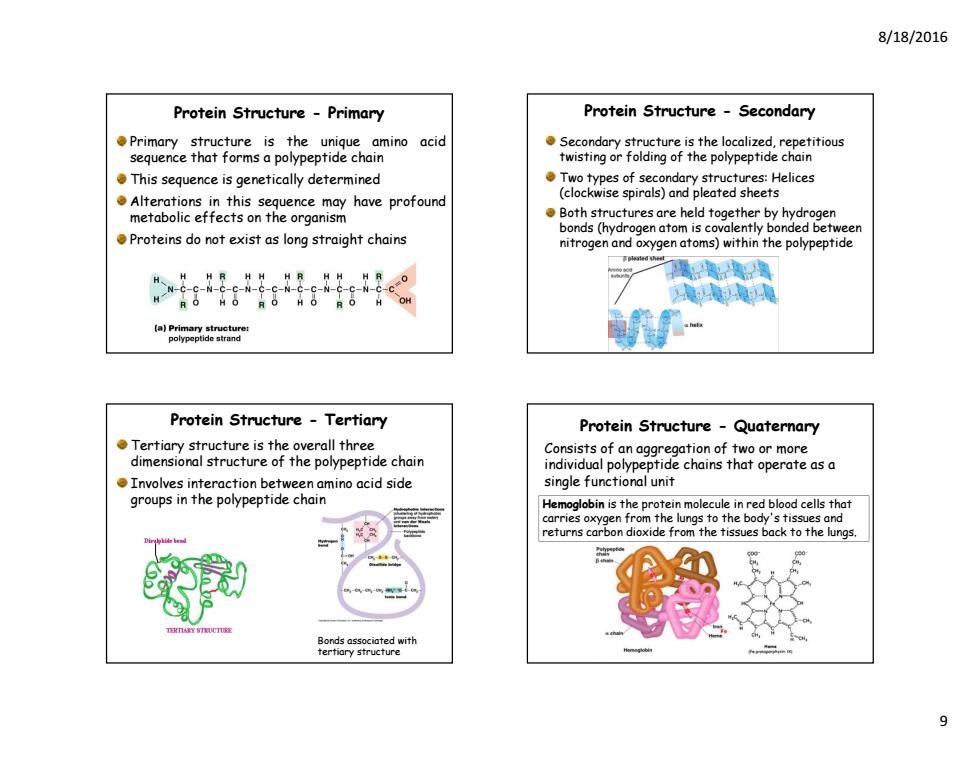
8/18/2016 Protein Structure-Primary Protein Structure -Secondary Primary structure is the unique amino acid Secondary structure is the localized,repetitious sequence that forms a polypeptide chain twisting or folding of the polypeptide chain This sequence is genetically determined Two types of secondary structures:Helices Alterations in this sequence may have profound (clockwise spirals)and pleated sheets metabolic effects on the organism Both structures are held together by hydrogen bonds(hydrogen atom is covalently bonded between Proteins do not exist as long straight chains nitrogen and oxygen atoms)within the polypeptide Protein Structure Tertiary Protein Structure-Quaternary Tertiary structure is the overall three Consists of an aggregation of two or more dimensional structure of the polypeptide chain individual polypeptide chains that operate asa Involves interaction between amino acid side single functional unit groups in the polypeptide chain Hemoglobin is the protein molecule in red blood cells that carries oxygen the ungs to the body's tissue and returns carbon dioxide from the tissues back to the lungs
8/18/2016 9 Protein Structure - Primary Primary structure is the unique amino acid sequence that forms a polypeptide chain This sequence is genetically determined Alterations in this sequence may have profound metabolic effects on the organism Proteins do not exist as long straight chains Protein Structure - Secondary Secondary structure is the localized, repetitious twisting or folding of the polypeptide chain Two types of secondary structures: Helices (clockwise spirals) and pleated sheets Both structures are held together by hydrogen bonds (hydrogen atom is covalently bonded between nitrogen and oxygen atoms) within the polypeptide Protein Structure - Tertiary Tertiary structure is the overall three dimensional structure of the polypeptide chain Involves interaction between amino acid side groups in the polypeptide chain Bonds associated with tertiary structure Protein Structure - Quaternary Consists of an aggregation of two or more individual polypeptide chains that operate as a single functional unit Hemoglobin is the protein molecule in red blood cells that carries oxygen from the lungs to the body's tissues and returns carbon dioxide from the tissues back to the lungs
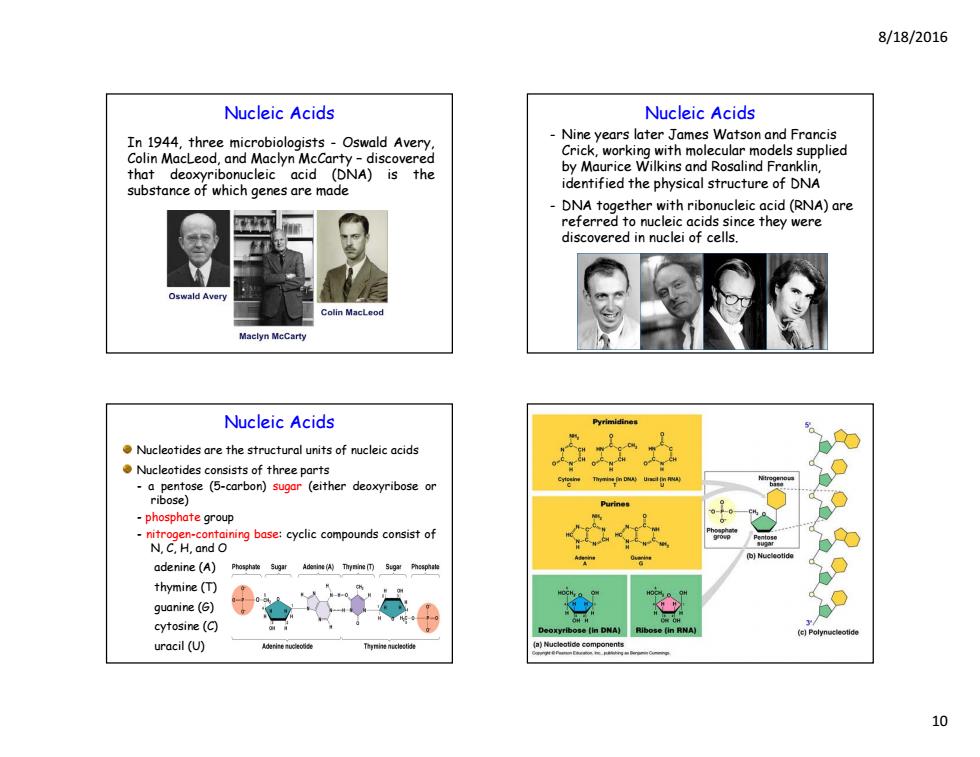
8/18/2016 Nucleic Acids Nucleic Acids In 1944,three microbiologists-Oswald Avery Nine years later James Watson and Francis Colin MacLeod,and Maclyn McCarty -discovered Crick,working with molecular models supplied that deoxyribonucleic acid (DNA)is the by Maurice Wilkins and Rosalind Franklin. substance of which genes are made identified the physical structure of DNA DNA together with ribonucleic acid(RNA)are referred to nucleic acids since they were discovered in nuclei of cells. Oswald Avery olin MacLeod Maclyn MeCarty Nucleic Acids Nucleotides are the structural units of nucleic acids Nucleotides consists of three parts ibenpose (-carbon)sugar (either deoxyribose or Purine -phosphate group adenine(A) Phoshae Sugir Adenlne (A)Thynine (Poaphale 60 thymine (T) guanine(G) cytosine(C) uracil (U) Tgnhnenucaet 10
8/18/2016 10 Nucleic Acids In 1944, three microbiologists - Oswald Avery, Colin MacLeod, and Maclyn McCarty – discovered that deoxyribonucleic acid (DNA) is the substance of which genes are made Nucleic Acids - Nine years later James Watson and Francis Crick, working with molecular models supplied by Maurice Wilkins and Rosalind Franklin, identified the physical structure of DNA - DNA together with ribonucleic acid (RNA) are referred to nucleic acids since they were discovered in nuclei of cells. Nucleic Acids Nucleotides are the structural units of nucleic acids Nucleotides consists of three parts - a pentose (5-carbon) sugar (either deoxyribose or ribose) - phosphate group - nitrogen-containing base: cyclic compounds consist of N, C, H, and O adenine (A) thymine (T) guanine (G) cytosine (C) uracil (U)