
Lecture 2 Energy and Energy Transfer
Energy and Energy Transfer Lecture 2
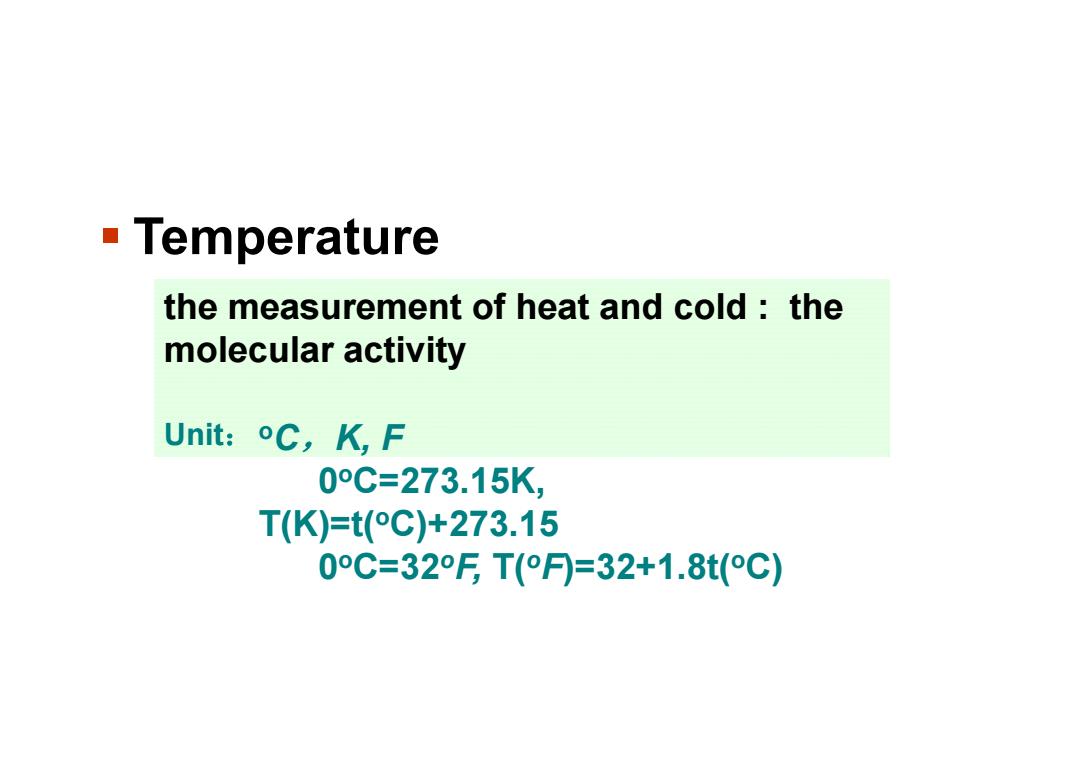
Temperature the measurement of heat and cold:the molecular activity Unit:oC,K,F 0°C=273.15K, T(K)=t(°C)+273.15 0°C=32FT(°月=32+1.8t(°C)
Temperature the measurement of heat and cold : the molecular activity Unit:oC,K, F 0oC=273.15K, T(K)=t(oC)+273.15 0oC=32oF, T(oF)=32+1.8t(oC)

Heat:Energy transferred solely due to temperature difference,Q
Heat: Energy transferred solely due to temperature difference, Q
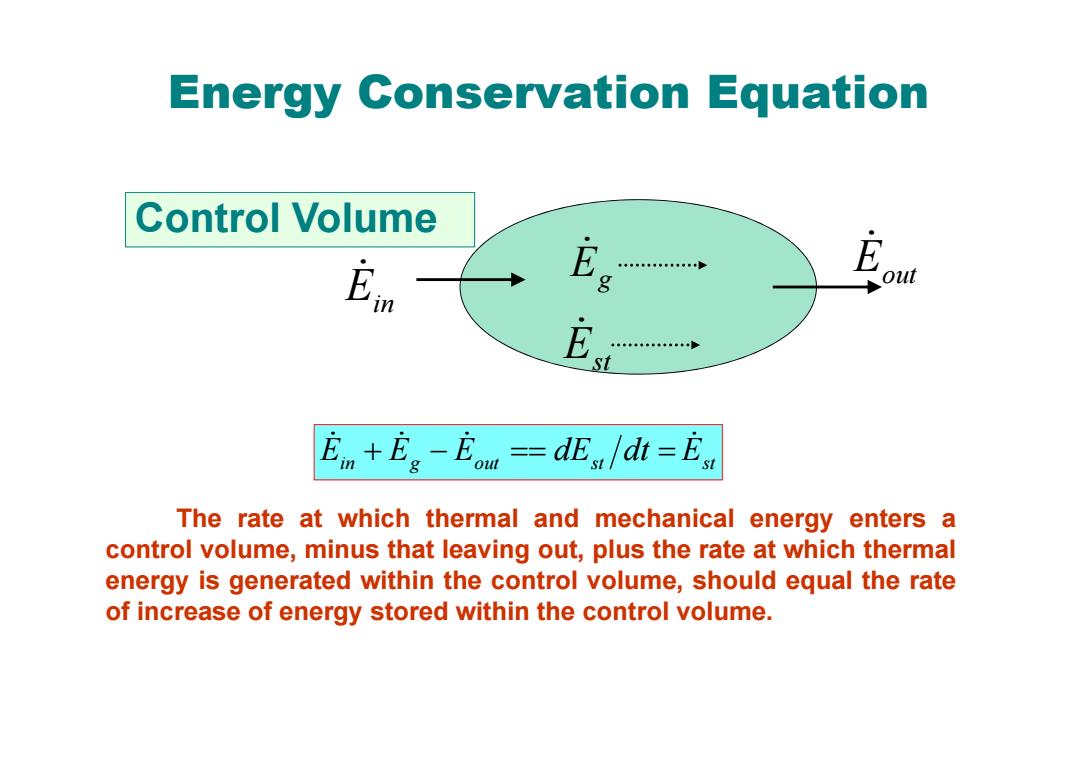
Energy Conservation Equation Control Volume 中年·”年… g out E t En+Eg-Eou =dEs /dt =Est The rate at which thermal and mechanical energy enters a control volume,minus that leaving out,plus the rate at which thermal energy is generated within the control volume,should equal the rate of increase of energy stored within the control volume
Energy Conservation Equation in g out st Est E E E dE dt The rate at which thermal and mechanical energy enters a control volume, minus that leaving out, plus the rate at which thermal energy is generated within the control volume, should equal the rate of increase of energy stored within the control volume. Control Volume Ein Eout E g Est
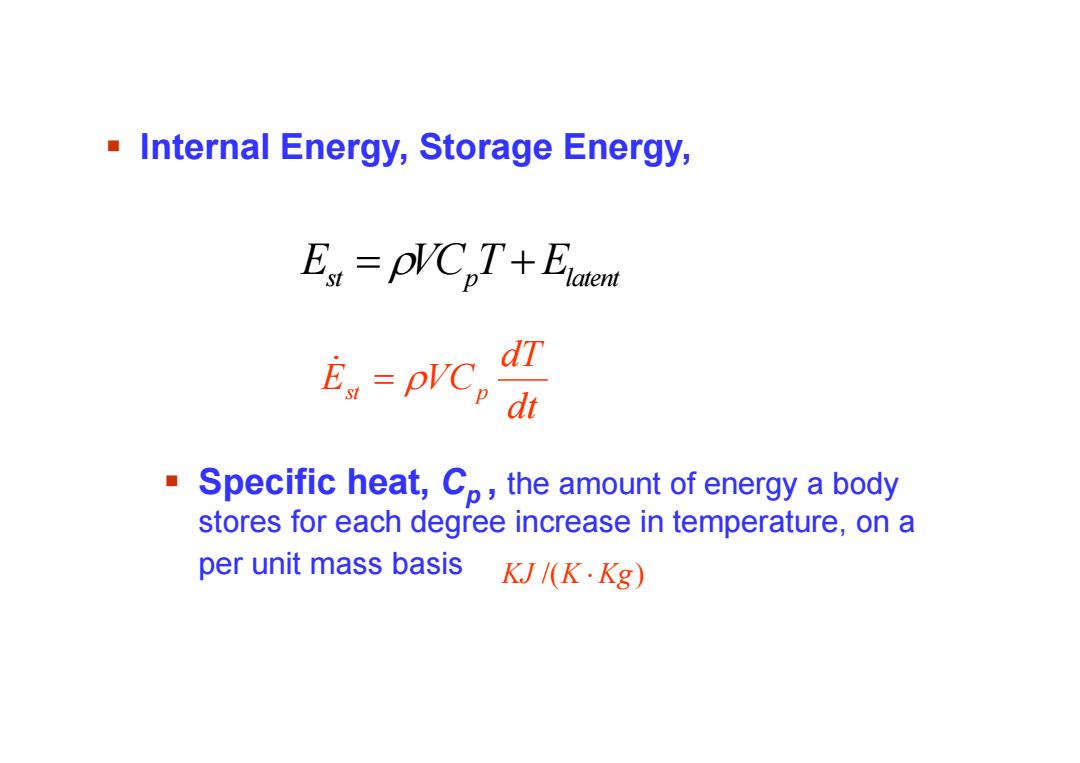
-Internal Energy,Storage Energy, E pVCpT+Elaten dT E=pr℃ dt ■ Specific heat,Co,the amount of energy a body stores for each degree increase in temperature,on a per unit mass basis KJ/(K.Kg)
Internal Energy, Storage Energy, dt dT Est VCp Specific heat, Cp , the amount of energy a body stores for each degree increase in temperature, on a per unit mass basis KJ /(K Kg) E VC T E st p latent
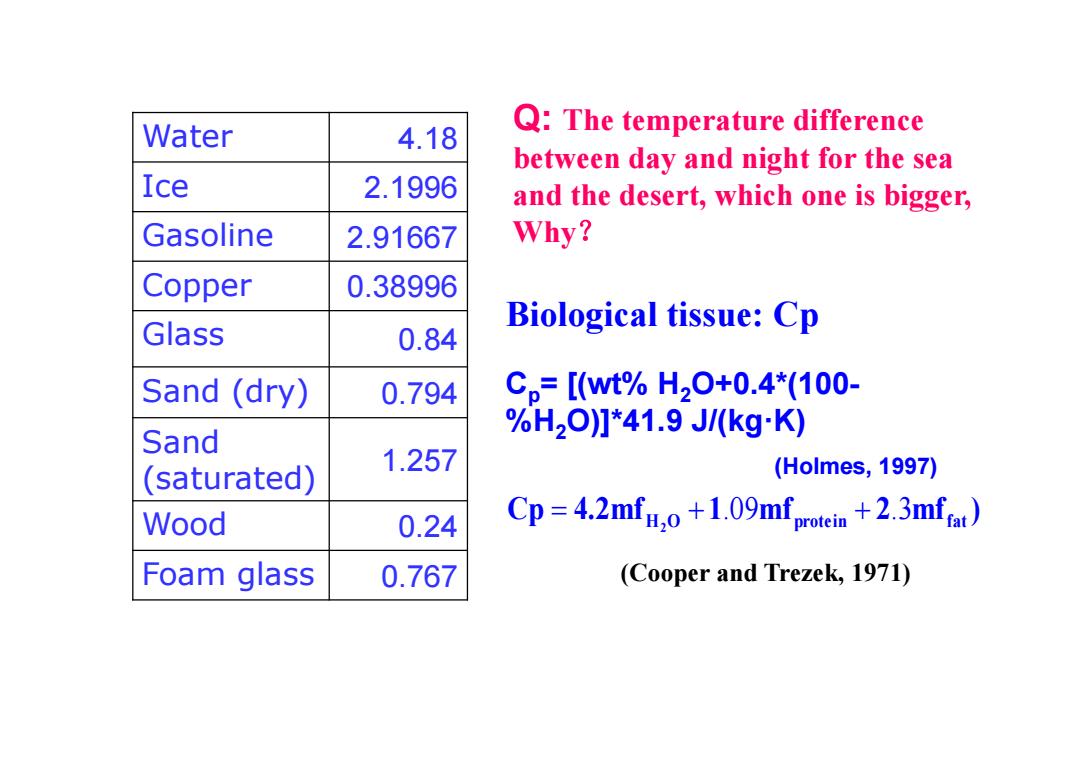
Water 4.18 Q:The temperature difference between day and night for the sea Ice 2.1996 and the desert,which one is bigger, Gasoline 2.91667 Why? Copper 0.38996 Glass Biological tissue:Cp 0.84 Sand (dry) 0.794 Cp=[wt%H20+0.4*(100- %H2O]*41.9J/(kgK) Sand 1.257 (saturated) (Holmes,1997) Wood 0.24 Cp=4.2mfo+1.09mfprotin +.3mfa Foam glass 0.767 (Cooper and Trezek,1971)
Water 4.18 Ice 2.1996 Gasoline 2.91667 Copper 0.38996 Glass 0.84 Sand (dry) 0.794 Sand (saturated) 1.257 Wood 0.24 Foam glass 0.767 Biological tissue: Cp Q: The temperature difference between day and night for the sea and the desert, which one is bigger, Why ? (Cooper and Trezek, 1971) Cp 4.2mf 1 mf 2 mf ) H O protein fat 2 .09 .3 C p= [(wt% H 2O+0.4*(100- %H 2O)]*41.9 J/(kg·K) (Holmes, 1997)

Volumetric Heat Generation in tissue q-gmet +ge MW External. RF Radiofrequency Tissue Metabolic heating Ultrasound, Activities Laser irradiation
q q met qe Tissue Metabolic Activities MW RF Radiofrequency Ultrasound, Laser irradiation …… External heating Volumetric Heat Generation in tissue
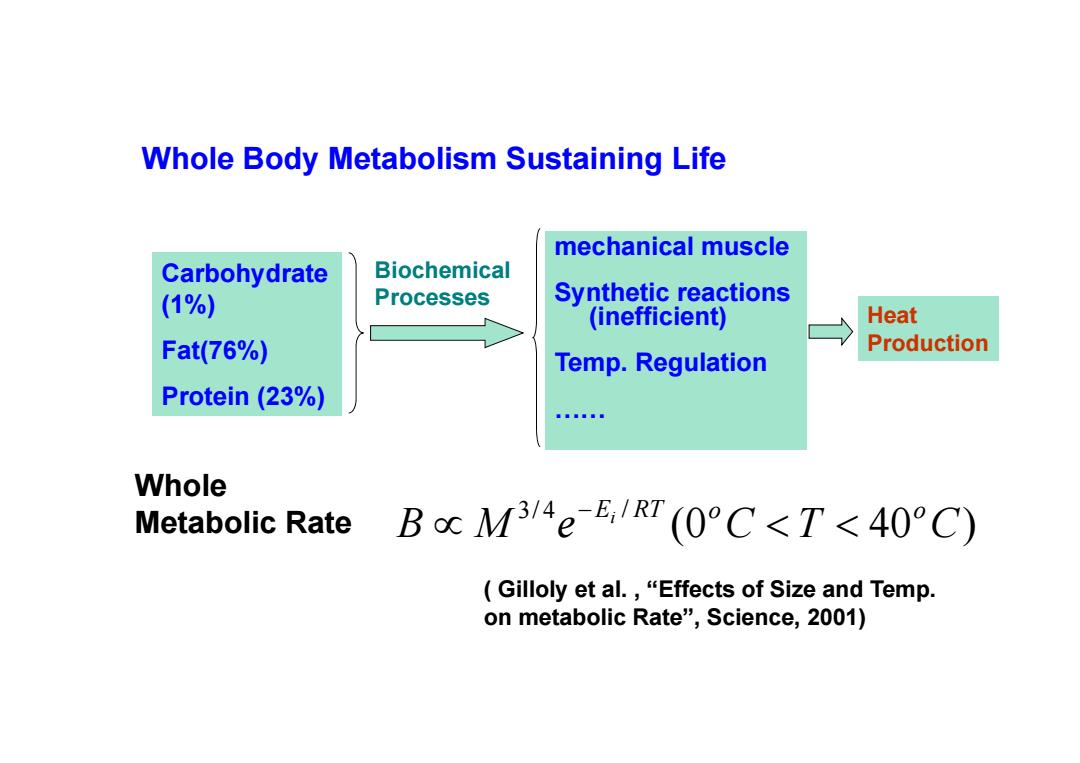
Whole Body Metabolism Sustaining Life mechanical muscle Carbohydrate Biochemical (1%) Processes Synthetic reactions (inefficient) Heat Fat(76%) Production Temp.Regulation Protein (23%) Bna。88 Whole Metabolic Rate BcM3/4eE,1RT(0°C<T<40°C) Gilloly et al.,"Effects of Size and Temp. on metabolic Rate",Science,2001)
mechanical muscle Synthetic reactions (inefficient) Temp. Regulation …… Carbohydrate (1%) Fat(76%) Protein (23%) Biochemical Processes Heat Production Whole Body Metabolism Sustaining Life Whole Metabolic Rate (0 40 ) 3/ 4 / B M e C T C Ei RT o o ( Gilloly et al. , “Effects of Size and Temp. on metabolic Rate”, Science, 2001)

M: Total mass E:Avg.activation energy for the rate limiting enzyme catalyzed biochemical reactions(0.2-1.2eV) R:Boltzman constant:1.38*10-23J/K-molecule Estimated from the oxidative metabolism (Pennes,1948) Skin ~0.17w/Kg ~170w/m3 Resting Skeletal Muscle ~170w/m3 Exercise Sekeletal Muscle ~10(20)*170w/m3 Tumor's metabolic rate may be higher!
M: Total mass Ei: Avg. activation energy for the rate limiting enzyme catalyzed biochemical reactions (0.2—1.2eV) R: Boltzman constant: 1.38*10-23J/K·molecule Estimated from the oxidative metabolism (Pennes, 1948) Skin ~ 0.17w/Kg ~ 170w/m3 Resting Skeletal Muscle ~ 170w/m3 Exercise Sekeletal Muscle ~ 10(20) * 170w/m3 Tumor’s metabolic rate may be higher!

Energy inflow and outflow by conduction,convection,radiation
Energy inflow and outflow by conduction, convection, radiation