
Hyperthermia treatment for tumors
Hyperthermia treatment for tumors
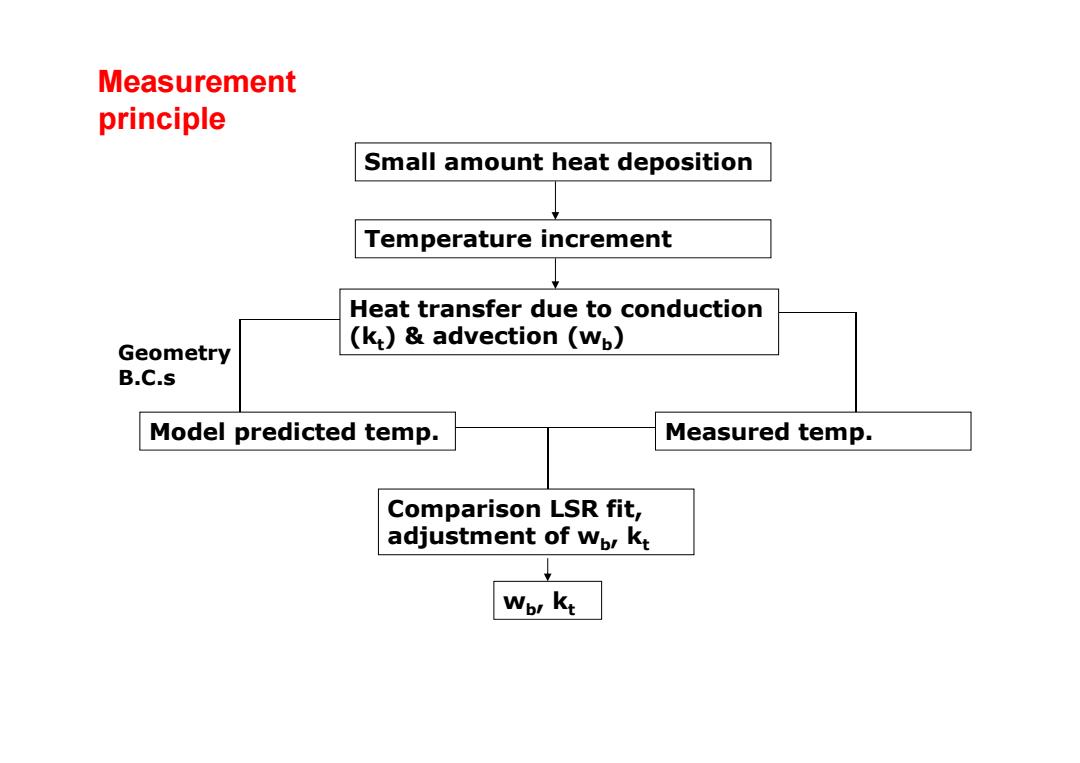
Measurement principle Small amount heat deposition Temperature increment Heat transfer due to conduction (k:)&advection (wp) Geometry B.C.s Model predicted temp. Measured temp. Comparison LSR fit, adjustment of wpr kt Wor kt
Small amount heat deposition Temperature increment Heat transfer due to conduction (k t) & advection (w b ) Model predicted temp. Measured temp. Comparison LSR fit, adjustment of w b, k t w b, k t Geometry B.C.s Measurement principle
Hyperthermia treatment for tumors 'Tumor Microenvironment Microvasculature Stage of tumor: Inhibitors 1.Dormant (no vasculature) 2.Vascularization (inducer:vascular growth factors) 3.Growth and Metastasis (new tumors) 4.Suppression of new tumors(inhibitors from main tumor), Necrotic(less perfusion in tumor center(in animal at least)- >average perfusion decrease)
Hyperthermia treatment for tumors •Tumor Microenvironment & Microvasculature • Stage of tumor: 1. Dormant (no vasculature) 2. Vascularization (inducer: vascular growth factors) 3. Growth and Metastasis (new tumors) 4. Suppression of new tumors (inhibitors from main tumor), Necrotic (less perfusion in tumor center (in animal at least) ‐ >average perfusion decrease)
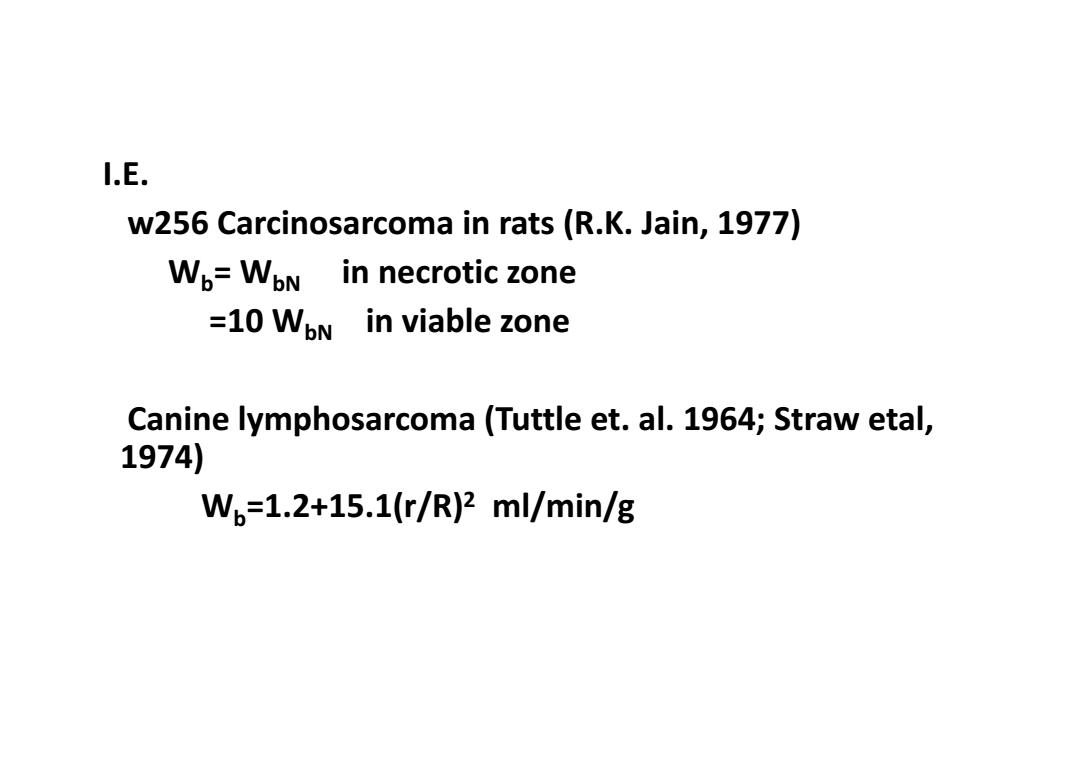
I.E. w256 Carcinosarcoma in rats (R.K.Jain,1977) Wo=WoN in necrotic zone =10 WoN in viable zone Canine lymphosarcoma(Tuttle et.al.1964;Straw etal, 1974) Wb=1.2+15.1(r/R)2ml/min/g
I.E. w256 Carcinosarcoma in rats (R.K. Jain, 1977) Wb= WbN in necrotic zone =10 WbN in viable zone Canine lymphosarcoma (Tuttle et. al. 1964; Straw etal, 1974) Wb=1.2+15.1(r/R)2 ml/min/g
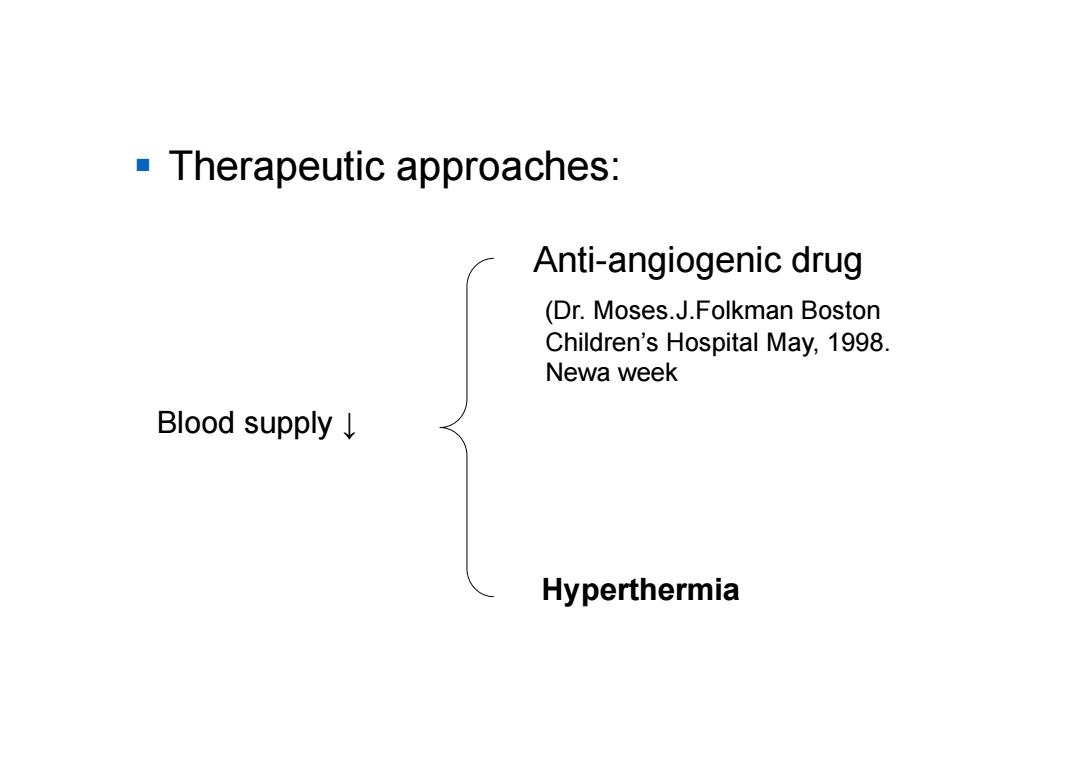
Therapeutic approaches: Anti-angiogenic drug (Dr.Moses.J.Folkman Boston Children's Hospital May,1998. Newa week Blood supply↓ Hyperthermia
Therapeutic approaches: Blood supply ↓ Anti-angiogenic drug (Dr. Moses.J.Folkman Boston Children’s Hospital May, 1998. Newa week Hyperthermia
Hyperthermia effect 1866(Busch):erysipelas (insect)attack >high fever>cure for a facial sarcoma 189l(Coley小:bacterial toxins→high fever(cancer patients) →reduced cancer size 1900:experiments done in lab animals>thermal dose (elevated temp.,duration)>effective treatment
Hyperthermia effect 1866(Busch): erysipelas (insect) aƩack →high fever → cure for a facial sarcoma 1891(Coley): bacterial toxins → high fever (cancer paƟents) → reduced cancer size 1900: experiments done in lab animals → thermal dose (elevated temp., duraƟon) → effecƟve treatment
1970s&1980s: Exp.studies at the cellular level> tumor cells are more sensitive to heat than normal cells due to abnormal molar ratios of cholesterol phospholipid Elevated temperature at 43C for a period of time is more lethal to tumor cells. cholesterol phospholipid↑→heat resistance of cells↑
1970s&1980s: Exp. studies at the cellular level → • tumor cells are more sensitive to heat than normal cells due to abnormal molar ratios of cholesterol phospholipid • Elevated temperature at 43Ԩ for a period of time is more lethal to tumor cells. cholesterol phospholipid↑ → heat resistance of cells ↑

1980s-present Numerous Exp Clinical trials Hyperthermia alone->partial reduction of tumor size Hyperthermia w/ionized (reduced hypoxia)radiation therapy better results Hyperthermia (increased circulation,increased vascular wall permeability to drugs)->better results
1980s‐present • Numerous Exp & Clinical trials • Hyperthermia alone‐>partial reduction of tumor size • Hyperthermia w/ ionized (reduced hypoxia) radiation therapy ‐ > better results • Hyperthermia (increased circulation, increased vascular wall permeability to drugs) ‐> better results
Effective Hyperthermia treatment a.Heating source selection: Surface Heating (local or whole body)-"Boundary condition" Hot water bath or moist air incubator ● Space suit and blanket techniques ● Infrared radiation ● Hot wax bath(molten paraffin wax having melting point of 46C) ● Regional systemic perfusion with heated blood Implantation of a heat source and electrocoagulation
Effective Hyperthermia treatment a. Heating source selection: Surface Heating (local or whole body)‐”Boundary condition” • Hot water bath or moist air incubator • Space suit and blanket techniques • Infrared radiation • Hot wax bath (molten paraffin wax having melting point of 46Ԩ) • Regional & systemic perfusion with heated blood • Implantation of a heat source and electrocoagulation

Volumetric heating (deep-seated tumors)-"q" ·Utrasonic:Sound wave→particle oscillation→mechanical energy converted to thermal energy by frictional losses λf=C 0.2MHz<f<3MHz C≈1500m/s sound speed in water For a plane-paralled beam:I Io exp(-ax) a attenuation coefficient ~fn(tissue,f) 1)Rapid absorption in bone-"hot spot" 2)Air doesn't allow transmission-not for tissue containing air:like chest cavity
Volumetric heating (deep‐seated tumors) –”q” • Utrasonic: Sound wave → parƟcle oscillaƟon → mechanical energy converted to thermal energy by frictional losses